Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids
- PMID: 23671091
- PMCID: PMC3670379
- DOI: 10.1073/pnas.1307237110
Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids
Abstract
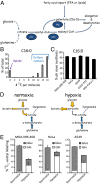
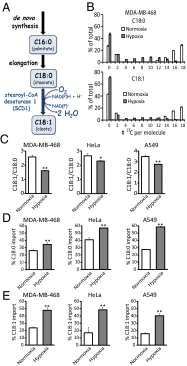
Cancer cell growth requires fatty acids to replicate cellular membranes. The kinase Akt is known to up-regulate fatty acid synthesis and desaturation, which is carried out by the oxygen-consuming enzyme stearoyl-CoA desaturase (SCD)1. We used (13)C tracers and lipidomics to probe fatty acid metabolism, including desaturation, as a function of oncogene expression and oxygen availability. During hypoxia, flux from glucose to acetyl-CoA decreases, and the fractional contribution of glutamine to fatty acid synthesis increases. In addition, we find that hypoxic cells bypass de novo lipogenesis, and thus, both the need for acetyl-CoA and the oxygen-dependent SCD1-reaction, by scavenging serum fatty acids. The preferred substrates for scavenging are phospholipids with one fatty acid tail (lysophospholipids). Hypoxic reprogramming of de novo lipogenesis can be reproduced in normoxic cells by Ras activation. This renders Ras-driven cells, both in culture and in allografts, resistant to SCD1 inhibition. Thus, a mechanism by which oncogenic Ras confers metabolic robustness is through lipid scavenging.
Keywords: isotope tracing; lipid metabolism; lipogenesis in cancer.
Conflict of interest statement
Conflict of interest statement: C.B.T. is a founder and consultant of Agios Pharmaceuticals and has a financial interest in Agios. C.B.T. is also on the Board of Directors of Merck.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

