Perineuronal nets protect fast-spiking interneurons against oxidative stress
- PMID: 23671099
- PMCID: PMC3670388
- DOI: 10.1073/pnas.1300454110
Perineuronal nets protect fast-spiking interneurons against oxidative stress
Abstract
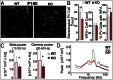
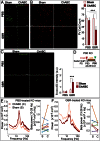
A hallmark of schizophrenia pathophysiology is the dysfunction of cortical inhibitory GABA neurons expressing parvalbumin, which are essential for coordinating neuronal synchrony during various sensory and cognitive tasks. The high metabolic requirements of these fast-spiking cells may render them susceptible to redox dysregulation and oxidative stress. Using mice carrying a genetic redox imbalance, we demonstrate that extracellular perineuronal nets, which constitute a specialized polyanionic matrix enwrapping most of these interneurons as they mature, play a critical role in the protection against oxidative stress. These nets limit the effect of genetically impaired antioxidant systems and/or excessive reactive oxygen species produced by severe environmental insults. We observe an inverse relationship between the robustness of the perineuronal nets around parvalbumin cells and the degree of intracellular oxidative stress they display. Enzymatic degradation of the perineuronal nets renders mature parvalbumin cells and fast rhythmic neuronal synchrony more susceptible to oxidative stress. In parallel, parvalbumin cells enwrapped with mature perineuronal nets are better protected than immature parvalbumin cells surrounded by less-condensed perineuronal nets. Although the perineuronal nets act as a protective shield, they are also themselves sensitive to excess oxidative stress. The protection might therefore reflect a balance between the oxidative burden on perineuronal net degradation and the capacity of the system to maintain the nets. Abnormal perineuronal nets, as observed in the postmortem patient brain, may thus underlie the vulnerability and functional impairment of pivotal inhibitory circuits in schizophrenia.
Keywords: critical period; extracellular matrix; glutamate cysteine ligase; glutathione; neuronal synchronization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Whittington MA, Cunningham MO, LeBeau FE, Racca C, Traub RD. Multiple origins of the cortical γ rhythm. Dev Neurobiol. 2011;71(1):92–106. - PubMed
-
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by N-acetylcysteine. Biol Psychiatry. 2013;73(6):574–582. - PubMed
-
- Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

