Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation
- PMID: 23671103
- PMCID: PMC3670396
- DOI: 10.1073/pnas.1306849110
Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation
Abstract
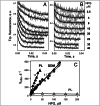
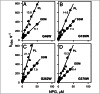
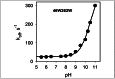
Trp replacements for conserved Gly-Gly pairs between the N- and C-terminal six-helix bundles on the periplasmic side of lactose permease (LacY) cause complete loss of transport activity with little or no effect on sugar binding. Moreover, the detergent-solubilized mutants exhibit much greater thermal stability than WT LacY. A Cys replacement for Asn245, which is inaccessible/unreactive in WT LacY, alkylates readily in the Gly→Trp mutants, indicating that the periplasmic cavity is patent. Stopped-flow kinetic measurements of sugar binding with the Gly→Trp mutants in detergent reveal linear dependence of binding rates on sugar concentration, as observed with WT or the C154G mutant of LacY, and are compatible with free access to the sugar-binding site in the middle of the molecule. Remarkably, after reconstitution of the Gly→Trp mutants into proteoliposomes, the concentration dependence of sugar-binding rates increases sharply with even faster rates than measured in detergent. Such behavior is strikingly different from that observed for reconstituted WT LacY, in which sugar-binding rates are independent of sugar concentration because opening of the periplasmic cavity is limiting for sugar binding. The observations clearly indicate that Gly→Trp replacements, which introduce bulky residues into tight Gly-Gly interdomain interactions on the periplasmic side of LacY, prevent closure of the periplasmic cavity and, as a result, shift the distribution of LacY toward an outward-open conformation.
Keywords: alternating access; fluorescence; major facilitator superfamily; permease; symport.
Figures


 ), G262W (■), G370W (◆), G46W/G262W (▼), G46W/G370W (★), or pT7-5 vector only with no LacY insert (○). Active sugar accumulation was measured at 0.4 mM [14C]-lactose, as described in Materials and Methods. One hundred percent transport corresponds to 160 nmol/mg protein. (B). Equilibrium exchange of lactose by RSO vesicles containing WT LacY (●), NEM-treated WT LacY (□), mutants with double Gly→Trp replacements G46W/G262W (▲), and G46W/G370W (▼), or no permease (○). RSO vesicles equilibrated with 10 mM [14C]lactose were diluted (1:200) into buffer containing 10 mM nonradioactive lactose and, at given times, radioactive lactose retained inside of vesicles was measured by rapid filtration as described in Materials and Methods. Nonspecific exchange of lactose in RSO vesicles containing WT LacY was assayed with an NEM-treated sample (see Materials and Methods for details).
), G262W (■), G370W (◆), G46W/G262W (▼), G46W/G370W (★), or pT7-5 vector only with no LacY insert (○). Active sugar accumulation was measured at 0.4 mM [14C]-lactose, as described in Materials and Methods. One hundred percent transport corresponds to 160 nmol/mg protein. (B). Equilibrium exchange of lactose by RSO vesicles containing WT LacY (●), NEM-treated WT LacY (□), mutants with double Gly→Trp replacements G46W/G262W (▲), and G46W/G370W (▼), or no permease (○). RSO vesicles equilibrated with 10 mM [14C]lactose were diluted (1:200) into buffer containing 10 mM nonradioactive lactose and, at given times, radioactive lactose retained inside of vesicles was measured by rapid filtration as described in Materials and Methods. Nonspecific exchange of lactose in RSO vesicles containing WT LacY was assayed with an NEM-treated sample (see Materials and Methods for details).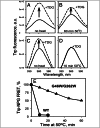

Similar articles
-
Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding.Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15147-51. doi: 10.1073/pnas.1112157108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896727 Free PMC article.
-
Outward-facing conformers of LacY stabilized by nanobodies.Proc Natl Acad Sci U S A. 2014 Dec 30;111(52):18548-53. doi: 10.1073/pnas.1422265112. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512549 Free PMC article.
-
Sugar binding induces an outward facing conformation of LacY.Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16504-9. doi: 10.1073/pnas.0708258104. Epub 2007 Oct 9. Proc Natl Acad Sci U S A. 2007. PMID: 17925435 Free PMC article.
-
The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport.FEBS Lett. 2003 Nov 27;555(1):96-101. doi: 10.1016/s0014-5793(03)01087-1. FEBS Lett. 2003. PMID: 14630326 Review.
-
The alternating access transport mechanism in LacY.J Membr Biol. 2011 Jan;239(1-2):85-93. doi: 10.1007/s00232-010-9327-5. Epub 2010 Dec 16. J Membr Biol. 2011. PMID: 21161516 Free PMC article. Review.
Cited by
-
New Horizons in Structural Biology of Membrane Proteins: Experimental Evaluation of the Role of Conformational Dynamics and Intrinsic Flexibility.Membranes (Basel). 2022 Feb 16;12(2):227. doi: 10.3390/membranes12020227. Membranes (Basel). 2022. PMID: 35207148 Free PMC article. Review.
-
It takes two to tango: The dance of the permease.J Gen Physiol. 2019 Jul 1;151(7):878-886. doi: 10.1085/jgp.201912377. Epub 2019 May 30. J Gen Physiol. 2019. PMID: 31147449 Free PMC article. Review.
-
A chemiosmotic mechanism of symport.Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1259-64. doi: 10.1073/pnas.1419325112. Epub 2015 Jan 7. Proc Natl Acad Sci U S A. 2015. PMID: 25568085 Free PMC article.
-
An Asymmetric Conformational Change in LacY.Biochemistry. 2017 Apr 4;56(13):1943-1950. doi: 10.1021/acs.biochem.7b00134. Epub 2017 Mar 23. Biochemistry. 2017. PMID: 28300394 Free PMC article.
-
Role of Conserved Gly-Gly Pairs on the Periplasmic Side of LacY.Biochemistry. 2016 Aug 9;55(31):4326-32. doi: 10.1021/acs.biochem.6b00666. Epub 2016 Aug 1. Biochemistry. 2016. PMID: 27438891 Free PMC article.
References
-
- Saier MH, Jr, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1(2):257–279. - PubMed
-
- Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35(4):699–710. - PubMed
-
- Patel L, Garcia ML, Kaback HR. Direct measurement of lactose/proton symport in Escherichia coli membrane vesicles: Further evidence for the involvement of histidine residue(s) Biochemistry. 1982;21(23):5805–5810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

