Dominant suppression of inflammation by glycan-hydrolyzed IgG
- PMID: 23671108
- PMCID: PMC3690874
- DOI: 10.1073/pnas.1301480110
Dominant suppression of inflammation by glycan-hydrolyzed IgG
Retraction in
-
Retraction for Nandakumar et al., Dominant suppression of inflammation by glycan-hydrolyzed IgG.Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15851. doi: 10.1073/pnas.1419095111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331904 Free PMC article. No abstract available.
Abstract
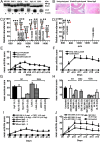
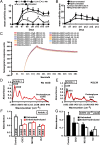
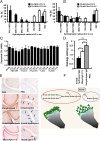
A unique anti-inflammatory property of IgG, independent of antigen specificity, is described. IgG with modification of the heavy-chain glycan on asparagine 297 by the streptococcal enzyme endo-β-N-acetylglucosaminidase (EndoS) induced a dominant suppression of immune complex (IC)-mediated inflammation, such as arthritis, through destabilization of local ICs by fragment crystallizable-fragment crystallizable (Fc-Fc) interactions. Small amounts (250 µg) of EndoS-hydrolyzed IgG were sufficient to inhibit arthritis in mice and most effective during the formation of ICs in the target tissue. The presence of EndoS-hydrolyzed IgG disrupted larger IC lattice formation both in vitro and in vivo, as visualized with anti-C3b staining. Neither complement binding in vitro nor antigen-antibody binding per se was affected.
Keywords: collagen; endoglycosidase; glycosylation; monoclonal antibody; rheumatoid arthritis.
Conflict of interest statement
Conflict of interest statement: Hansa Medical AB has filed patent applications for using EndoS-modified IgG for treatment of arthritis and K.S.N., M.C., and R.H. are listed as inventors. The authors have no additional financial interests. The funders had no role in the preparation of the manuscript or the decision to publish.
Figures
Comment in
-
Therapeutic potential of deglycosylated antibodies.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10059-60. doi: 10.1073/pnas.1307776110. Epub 2013 Jun 7. Proc Natl Acad Sci U S A. 2013. PMID: 23749872 Free PMC article. No abstract available.
References
-
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20(9):2361–2370. - PubMed
-
- Parekh RB, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. - PubMed
-
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

