Systematic identification of conserved bacterial c-di-AMP receptor proteins
- PMID: 23671116
- PMCID: PMC3670340
- DOI: 10.1073/pnas.1300595110
Systematic identification of conserved bacterial c-di-AMP receptor proteins
Abstract
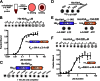
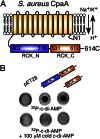
Nucleotide signaling molecules are important messengers in key pathways that allow cellular responses to changing environments. Canonical secondary signaling molecules act through specific receptor proteins by direct binding to alter their activity. Cyclic diadenosine monophosphate (c-di-AMP) is an essential signaling molecule in bacteria that has only recently been discovered. Here we report on the identification of four Staphylococcus aureus c-di-AMP receptor proteins that are also widely distributed among other bacteria. Using an affinity pull-down assay we identified the potassium transporter-gating component KtrA as a c-di-AMP receptor protein, and it was further shown that this protein, together with c-di-AMP, enables S. aureus to grow in low potassium conditions. We defined the c-di-AMP binding activity within KtrA to the RCK_C (regulator of conductance of K(+)) domain. This domain is also found in a second S. aureus protein, a predicted cation/proton antiporter, CpaA, which as we show here also directly binds c-di-AMP. Because RCK_C domains are found in proteinaceous channels, transporters, and antiporters from all kingdoms of life, these findings have broad implications for the regulation of different pathways through nucleotide-dependent signaling. Using a genome-wide nucleotide protein interaction screen we further identified the histidine kinase protein KdpD that in many bacteria is also involved in the regulation of potassium transport and a PII-like signal transduction protein, which we renamed PstA, as c-di-AMP binding proteins. With the identification of these widely distributed c-di-AMP receptor proteins we link the c-di-AMP signaling network to a central metabolic process in bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dalebroux ZD, Swanson MS. ppGpp: Magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10(3):203–212. - PubMed
-
- Witte G, Hartung S, Büttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 2008;30(2):167–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

