Mutagenicity and tumorigenicity of the four enantiopure bay-region 3,4-diol-1,2-epoxide isomers of dibenz[a,h]anthracene
- PMID: 23671133
- PMCID: PMC3765047
- DOI: 10.1093/carcin/bgt164
Mutagenicity and tumorigenicity of the four enantiopure bay-region 3,4-diol-1,2-epoxide isomers of dibenz[a,h]anthracene
Abstract
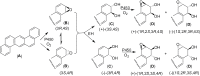
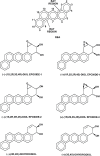
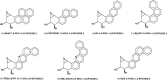
Each enantiomer of the diastereomeric pair of bay-region dibenz[a,h]anthracene 3,4-diol-1,2-epoxides in which the benzylic 4-hydroxyl group and epoxide oxygen are either cis (isomer 1) or trans (isomer 2) were evaluated for mutagenic activity. In strains TA 98 and TA 100 of Salmonella typhimurium, the diol epoxide with (1S,2R,3S,4R) absolute configuration [(-)-diol epoxide-1] had the highest mutagenic activity. In Chinese hamster V-79 cells, the diol epoxide with (1R,2S,3S,4R) absolute configuration [(+)-diol epoxide-2] had the highest mutagenic activity. The (1R,2S,3R,4S) diol epoxide [(+)-diol epoxide-1] also had appreciable activity, whereas the other two bay-region diol epoxide enantiomers had very low activity. In tumor studies, the (1R,2S,3S,4R) enantiomer was the only diol epoxide isomer tested that had strong activity as a tumor initiator on mouse skin and in causing lung and liver tumors when injected into newborn mice. This stereoisomer was about one-third as active as the parent hydrocarbon, dibenz[a,h]anthracene as a tumor initiator on mouse skin; it was several-fold more active than dibenz[a,h]anthracene as a lung and liver carcinogen when injected into newborn mice. (-)-(3R,4R)-3β,4α-dihydroxy-3,4-dihydro-dibenz[a,h]anthracene [(-)-3,4-dihydrodiol] was slightly more active than dibenz[a,h]anthracene as a tumor initiator on mouse skin, whereas (+)-(3S,4S)-3α,4β-dihydroxy-3,4-dihydro-dibenz[a,h]anthracene [(+)-3,4-dihydrodiol] had only very weak activity. The present investigation and previous studies with the corresponding four possible enantiopure bay-region diol epoxide enantiomers/diastereomers of benzo[a]pyrene, benz[a]anthracene, chrysene, benzo[c]phenanthrene, dibenz[c,h]acridine, dibenz[a,h]acridine and dibenz[a,h]anthracene indicate that the bay-region diol epoxide enantiomer with [R,S,S,R] absolute stereochemistry has high tumorigenic activity on mouse skin and in newborn mice.
Figures
References
-
- Jerina D.M, et al. (1974). Arene oxides: a new aspect of drug metabolism. Science, 185, 573–582 - PubMed
-
- Miller E.C, et al. (1974). Biochemical mechanisms of chemical carcinognesisIn Bush H. (ed.) Molecular Biology of Cancer, Academic Press, New York, pp. 377–402
-
- Miller J.A. (1970). Carcinogenesis by chemicals: an overview–G. H. A. Clowes memorial lecture. Cancer Res., 30, 559–576 - PubMed
-
- Sims P, et al. (1974). Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. Adv. Cancer Res., 20, 165–274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

