Paired-pulse facilitation at recurrent Purkinje neuron synapses is independent of calbindin and parvalbumin during high-frequency activation
- PMID: 23671160
- PMCID: PMC3717232
- DOI: 10.1113/jphysiol.2013.254128
Paired-pulse facilitation at recurrent Purkinje neuron synapses is independent of calbindin and parvalbumin during high-frequency activation
Abstract
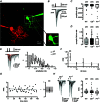
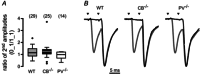
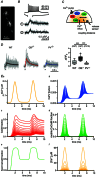
Paired-pulse facilitation (PPF) is a dynamic enhancement of transmitter release considered crucial in CNS information processing. The mechanisms of PPF remain controversial and may differ between synapses. Endogenous Ca(2+) buffers such as parvalbumin (PV) and calbindin-D28k (CB) are regarded as important modulators of PPF, with PV acting as an anti-facilitating buffer while saturation of CB can promote PPF. We analysed transmitter release and PPF at intracortical, recurrent Purkinje neuron (PN) to PN synapses, which show PPF during high-frequency activation (200 Hz) and strongly express both PV and CB. We quantified presynaptic Ca(2+) dynamics and quantal release parameters in wild-type (WT), and CB and PV deficient mice. Lack of CB resulted in increased volume averaged presynaptic Ca(2+) amplitudes and in increased release probability, while loss of PV had no significant effect on these parameters. Unexpectedly, none of the buffers significantly influenced PPF, indicating that neither CB saturation nor residual free Ca(2+) ([Ca(2+)]res) was the main determinant of PPF. Experimentally constrained, numerical simulations of Ca(2+)-dependent release were used to estimate the contributions of [Ca(2+)]res, CB, PV, calmodulin (CaM), immobile buffer fractions and Ca(2+) remaining bound to the release sensor after the first of two action potentials ('active Ca(2+)') to PPF. This analysis indicates that PPF at PN-PN synapses does not result from either buffer saturation or [Ca(2+)]res but rather from slow Ca(2+) unbinding from the release sensor.
Figures
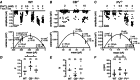

Comment in
-
Short-term synaptic plasticity and the 'active calcium' hypothesis at a central synapse.J Physiol. 2013 Oct 1;591(19):4681-2. doi: 10.1113/jphysiol.2013.258590. J Physiol. 2013. PMID: 24085489 Free PMC article. No abstract available.
References
-
- Bertram R, Sherman A, Stanley EF. Single-domain/bound calcium hypothesis of transmitter release and facilitation. J Neurophysiol. 1996;75:1919–1931. - PubMed
-
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

