Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction
- PMID: 23671276
- PMCID: PMC3696697
- DOI: 10.1074/jbc.M112.445445
Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction
Abstract
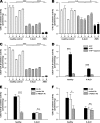
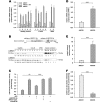
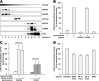
X-linked adrenoleukodystrophy (X-ALD), an inherited peroxisomal disorder, is caused by mutations in the ABCD1 gene encoding the peroxisomal ATP-binding cassette (ABC) transporter ABCD1 (adrenoleukodystrophy protein, ALDP). Biochemically, X-ALD is characterized by an accumulation of very long-chain fatty acids and partially impaired peroxisomal β-oxidation. In this study, we used primary human fibroblasts from X-ALD and Zellweger syndrome patients to investigate the peroxisomal β-oxidation defect. Our results show that the degradation of C26:0-CoA esters is as severely impaired as degradation of unesterified very long-chain fatty acids in X-ALD and is abolished in Zellweger syndrome. Interestingly, the β-oxidation rates for both C26:0-CoA and C22:0-CoA were similarly affected, although C22:0 does not accumulate in patient fibroblasts. Furthermore, we show that the β-oxidation defect in X-ALD is directly caused by ABCD1 dysfunction as blocking ABCD1 function with a specific antibody reduced β-oxidation to levels observed in X-ALD fibroblasts. By quantification of mRNA and protein levels of the peroxisomal ABC transporters and by blocking with specific antibodies, we found that residual β-oxidation activity toward C26:0-CoA in X-ALD fibroblasts is mediated by ABCD3, although the efficacy of ABCD3 appeared to be much lower than that of ABCD1. Finally, using isolated peroxisomes, we show that β-oxidation of C26:0-CoA is independent of additional CoA but requires a cytosolic factor of >10-kDa molecular mass that is resistant to N-ethylmaleimide and heat inactivation. In conclusion, our findings in human cells suggest that, in contrast to yeast cells, very long-chain acyl-CoA esters are transported into peroxisomes by ABCD1 independently of additional synthetase activity.
Keywords: ABC Transporter; ABCD1; ABCD3; Adrenoleukodystrophy Protein; Fatty Acid Transport; Metabolic Diseases; Peroxisomes; VLCFA; X-ALD; β-Oxidation.
Figures


References
-
- Bezman L., Moser A. B., Raymond G. V., Rinaldo P., Watkins P. A., Smith K. D., Kass N. E., Moser H. W. (2001) Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 49, 512–517 - PubMed
-
- Kemp S., Berger J., Aubourg P. (2012) X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim. Biophys. Acta 1822, 1465–1474 - PubMed
-
- Moser A. B., Moser H. W. (1999) The prenatal diagnosis of X-linked adrenoleukodystrophy. Prenat. Diagn. 19, 46–48 - PubMed
-
- Schutgens R. B., Bouman I. W., Nijenhuis A. A., Wanders R. J., Frumau M. E. (1993) Profiles of very-long-chain fatty acids in plasma, fibroblasts, and blood cells in Zellweger syndrome, X-linked adrenoleukodystrophy, and rhizomelic chondrodysplasia punctata. Clin. Chem. 39, 1632–1637 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

