Human rotavirus VP6-specific antibodies mediate intracellular neutralization by binding to a quaternary structure in the transcriptional pore
- PMID: 23671563
- PMCID: PMC3650007
- DOI: 10.1371/journal.pone.0061101
Human rotavirus VP6-specific antibodies mediate intracellular neutralization by binding to a quaternary structure in the transcriptional pore
Abstract
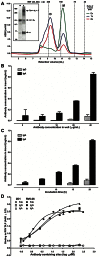
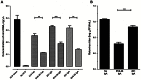
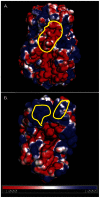
Several live attenuated rotavirus (RV) vaccines have been licensed, but the mechanisms of protective immunity are still poorly understood. The most frequent human B cell response is directed to the internal protein VP6 on the surface of double-layered particles, which is normally exposed only in the intracellular environment. Here, we show that the canonical VP6 antibodies secreted by humans bind to such particles and inhibit viral transcription. Polymeric IgA RV antibodies mediated an inhibitory effect against virus replication inside cells during IgA transcytosis. We defined the recognition site on VP6 as a quaternary epitope containing a high density of charged residues. RV human mAbs appear to bind to a negatively-charged patch on the surface of the Type I channel in the transcriptionally active particle, and they sterically block the channel. This unique mucosal mechanism of viral neutralization, which is not apparent from conventional immunoassays, may contribute significantly to human immunity to RV.
Conflict of interest statement
Figures




References
-
- Estes MK (2001) Rotavirus and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams and Wilkins. pp. 1747–1785.
-
- Kapikian A (2001) A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal. Novartis Found Symp 238: 153–171 (Discussion, 238:171–179.). - PubMed
-
- Prasad BVV, Rothnagel R, Zeng CQY, Jakana J, Lawton JA, et al. (1996) Visualization of ordered genomic RNA and localization of transcriptional complexes in rotavirus. Nature 382: 471–473. - PubMed
-
- Estes MK, Kapikian AZ (2007) Rotaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams and Wilkins. pp. 1918–1974.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

