Comprehensive antigen screening identifies Moraxella catarrhalis proteins that induce protection in a mouse pulmonary clearance model
- PMID: 23671716
- PMCID: PMC3650003
- DOI: 10.1371/journal.pone.0064422
Comprehensive antigen screening identifies Moraxella catarrhalis proteins that induce protection in a mouse pulmonary clearance model
Abstract
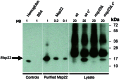
Moraxella catarrhalis is one of the three most common causative bacterial pathogens of otitis media, however no effective vaccine against M. catarrhalis has been developed so far. To identify M. catarrhalis vaccine candidate antigens, we used carefully selected sera from children with otitis media and healthy individuals to screen small-fragment genomic libraries that are expressed to display frame-selected peptides on a bacterial cell surface. This ANTIGENome technology led to the identification of 214 antigens, 23 of which were selected by in vitro or in vivo studies for additional characterization. Eight of the 23 candidates were tested in a Moraxella mouse pulmonary clearance model, and 3 of these antigens induced significantly faster bacterial clearance compared to adjuvant or to the previously characterized antigen OmpCD. The most significant protection data were obtained with the antigen MCR_1416 (Msp22), which was further investigated for its biological function by in vitro studies suggesting that Msp22 is a heme binding protein. This study comprises one of the most exhaustive studies to identify potential vaccine candidate antigens against the bacterial pathogen M. catarrhalis.
Conflict of interest statement
Figures

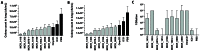

References
-
- Enright MC, McKenzie H (1997) Moraxella (Branhamella) catarrhalis – clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol 46: 360–371. - PubMed
-
- Murphy TF, Bakaletz LO, Kyd JM, Watson B, Klein DL (2005) Vaccines for otitis media: proposals for overcoming obstacles to progress. Vaccine 23: 2696–2702. - PubMed
-
- Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM (2004) Otitis media. Lancet 363: 465–473. - PubMed
-
- Cripps AW, Otczyk DC (2006) Prospects for a vaccine against otitis media. Expert Rev Vaccines 5: 517–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

