Kinetic characterization of hydrolysis of nitrocefin, cefoxitin, and meropenem by β-lactamase from Mycobacterium tuberculosis
- PMID: 23672214
- PMCID: PMC3750105
- DOI: 10.1021/bi400177y
Kinetic characterization of hydrolysis of nitrocefin, cefoxitin, and meropenem by β-lactamase from Mycobacterium tuberculosis
Abstract
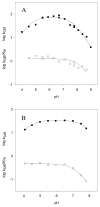
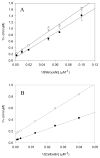
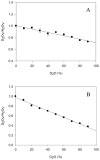
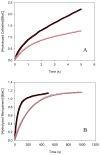
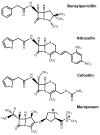
The constitutively expressed, chromosomally encoded β-lactamase (BlaC) is the enzyme responsible for the intrinsic resistance to β-lactam antibiotics in Mycobacterium tuberculosis. Previous studies from this laboratory have shown that the enzyme exhibits an extended-spectrum phenotype, with very high levels of penicillinase and cephalosporinase activity, as well as weak carbapenemase activity [Tremblay, L. W., et al. (2008) Biochemistry 47, 5312-5316]. In this report, we have determined the pH dependence of the kinetic parameters, revealing that the maximal velocity depends on the ionization state of two groups: a general base exhibiting a pK value of 4.5 and a general acid exhibiting a pK value of 7.8. Having defined a region where the kinetic parameters are pH-independent (pH 6.5), we determined solvent kinetic isotope effects (SKIEs) for three substrates whose kcat values differ by 5.5 orders of magnitude. Nitrocefin is a highly activated, chromogenic cephalosporin derivative that exhibits steady-state solvent kinetic isotope effects of 1.4 on both V and V/K. Cefoxitin is a slower cephalosporin derivative that exhibits a large SKIE on V of 3.9 but a small SKIE of 1.8 on V/K in steady-state experiments. Pre-steady-state, stopped-flow experiments with cefoxitin revealed a burst of β-lactam ring opening with associated SKIE values of 1.6 on the acylation step and 3.4 on the deacylation step. Meropenem is an extremely slow substrate for BlaC and exhibits burst kinetics in the steady-state experiments. SKIE determinations with meropenem revealed large SKIEs on both the acylation and deacylation steps of 3.8 and 4.0, respectively. Proton inventories in all cases were linear, indicating the participation of a single solvent-derived proton in the chemical step responsible for the SKIE. The rate-limiting steps for β-lactam hydrolysis of these substrates are analyzed, and the chemical steps responsible for the observed SKIE are discussed.
Figures
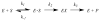
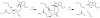
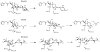
Similar articles
-
Chemical mechanism of a cysteine protease, cathepsin C, as revealed by integration of both steady-state and pre-steady-state solvent kinetic isotope effects.Biochemistry. 2008 Aug 19;47(33):8697-710. doi: 10.1021/bi8007627. Epub 2008 Jul 26. Biochemistry. 2008. PMID: 18656960
-
Kinetic and Structural Characterization of the Interaction of 6-Methylidene Penem 2 with the β-Lactamase from Mycobacterium tuberculosis.Biochemistry. 2015 Sep 15;54(36):5657-64. doi: 10.1021/acs.biochem.5b00698. Epub 2015 Aug 31. Biochemistry. 2015. PMID: 26237118 Free PMC article.
-
The amino-acid substituents of dipeptide substrates of cathepsin C can determine the rate-limiting steps of catalysis.Biochemistry. 2012 Sep 25;51(38):7551-68. doi: 10.1021/bi300719b. Epub 2012 Sep 13. Biochemistry. 2012. PMID: 22928782
-
Beta-secondary and solvent deuterium kinetic isotope effects on beta-lactamase catalysis.Biochemistry. 1996 Mar 19;35(11):3604-13. doi: 10.1021/bi952107i. Biochemistry. 1996. PMID: 8639512
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
Cited by
-
Adding Insult to Injury: Mechanistic Basis for How AmpC Mutations Allow Pseudomonas aeruginosa To Accelerate Cephalosporin Hydrolysis and Evade Avibactam.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00894-20. doi: 10.1128/AAC.00894-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32660987 Free PMC article.
-
A Novel Metallo-β-Lactamase Involved in the Ampicillin Resistance of Streptococcus pneumoniae ATCC 49136 Strain.PLoS One. 2016 May 23;11(5):e0155905. doi: 10.1371/journal.pone.0155905. eCollection 2016. PLoS One. 2016. PMID: 27214294 Free PMC article.
-
Insights into the catalytic mechanism of a bacterial hydrolytic dehalogenase that degrades the fungicide chlorothalonil.J Biol Chem. 2019 Sep 6;294(36):13411-13420. doi: 10.1074/jbc.RA119.009094. Epub 2019 Jul 21. J Biol Chem. 2019. PMID: 31331935 Free PMC article.
-
Biochemical Insights into Imipenem Collateral Susceptibility Driven by ampC Mutations Conferring Ceftolozane/Tazobactam Resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2023 Feb 16;67(2):e0140922. doi: 10.1128/aac.01409-22. Epub 2023 Jan 30. Antimicrob Agents Chemother. 2023. PMID: 36715512 Free PMC article.
-
Antibacterial Spectrum of a Tetrazole-Based Reversible Inhibitor of Serine β-Lactamases.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e02563-17. doi: 10.1128/AAC.02563-17. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29844038 Free PMC article.
References
-
- Tremblay LW, Hugonnet JE, Blanchard JS. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry. 2008;47:5312–5316. - PubMed
-
- TB Alliance. The 2010 Annual Report for TB Drug Development (online) 2010 http://www.tballiance.org/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

