Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung
- PMID: 23672262
- PMCID: PMC3824047
- DOI: 10.1165/rcmb.2013-0086MA
Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung
Abstract
The lung hosts multiple populations of macrophages and dendritic cells, which play a crucial role in lung pathology. The accurate identification and enumeration of these subsets are essential for understanding their role in lung pathology. Flow cytometry is a mainstream tool for studying the immune system. However, a systematic flow cytometric approach to identify subsets of macrophages and dendritic cells (DCs) accurately and consistently in the normal mouse lung has not been described. Here we developed a panel of surface markers and an analysis strategy that accurately identify all known populations of macrophages and DCs, and their precursors in the lung during steady-state conditions and bleomycin-induced injury. Using this panel, we assessed the polarization of lung macrophages during the course of bleomycin-induced lung injury. Alveolar macrophages expressed markers of alternatively activated macrophages during both acute and fibrotic phases of bleomycin-induced lung injury, whereas markers of classically activated macrophages were expressed only during the acute phase. Taken together, these data suggest that this flow cytometric panel is very helpful in identifying macrophage and DC populations and their state of activation in normal, injured, and fibrotic lungs.
Figures
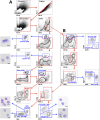

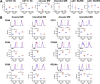
References
-
- Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012;129:889–901. - PubMed
-
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. - PubMed
-
- Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES013995/ES/NIEHS NIH HHS/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- HL071643/HL/NHLBI NIH HHS/United States
- AR054796/AR/NIAMS NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- AR050250/AR/NIAMS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- R21 AI092490/AI/NIAID NIH HHS/United States
- R01 AR050250/AR/NIAMS NIH HHS/United States
- R01 AR054796/AR/NIAMS NIH HHS/United States
- AI092490/AI/NIAID NIH HHS/United States
- ES015024/ES/NIEHS NIH HHS/United States
- P01 HL108795/HL/NHLBI NIH HHS/United States
- HL108795/HL/NHLBI NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 AR064546/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

