Deriving cell lines from zebrafish embryos and tumors
- PMID: 23672287
- PMCID: PMC3760073
- DOI: 10.1089/zeb.2013.0866
Deriving cell lines from zebrafish embryos and tumors
Abstract
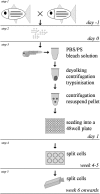
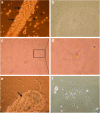
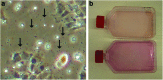
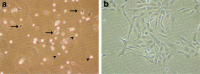
Over the last two decades the zebrafish has emerged as a powerful model organism in science. The experimental accessibility, the broad range of zebrafish mutants, and the highly conserved genetic and biochemical pathways between zebrafish and mammals lifted zebrafish to become one of the most attractive vertebrate models to study gene function and to model human diseases. Zebrafish cell lines are highly attractive to investigate cell biology and zebrafish cell lines complement the experimental tools that are available already. We established a straightforward method to culture cells from a single zebrafish embryo or a single tumor. Here we describe the generation of fibroblast-like cell lines from wild-type and ptenb(-/-) embryos and an endothelial-like cell line from a tumor of an adult ptena(+/-)ptenb(-/-) zebrafish. This protocol can easily be adapted to establish stable cell lines from any mutant or transgenic zebrafish line and the average time to obtain a pro-stable cell line is 3-5 months.
Figures


References
-
- Lieschke GJ. Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. - PubMed
-
- Amatruda JF. Shepard JL. Stern HM. Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–231. - PubMed
-
- Alders M. Hogan BM. Gjini E. Salehi F. Al-Gazali L. Hennekam EA, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41:1272–1274. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

