Insights into the phosphoryl transfer catalyzed by cAMP-dependent protein kinase: an X-ray crystallographic study of complexes with various metals and peptide substrate SP20
- PMID: 23672593
- PMCID: PMC3666212
- DOI: 10.1021/bi400066a
Insights into the phosphoryl transfer catalyzed by cAMP-dependent protein kinase: an X-ray crystallographic study of complexes with various metals and peptide substrate SP20
Abstract
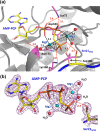
X-ray structures of several ternary substrate and product complexes of the catalytic subunit of cAMP-dependent protein kinase (PKAc) have been determined with different bound metal ions. In the PKAc complexes, Mg(2+), Ca(2+), Sr(2+), and Ba(2+) metal ions could bind to the active site and facilitate the phosphoryl transfer reaction. ATP and a substrate peptide (SP20) were modified, and the reaction products ADP and the phosphorylated peptide were found trapped in the enzyme active site. Finally, we determined the structure of a pseudo-Michaelis complex containing Mg(2+), nonhydrolyzable AMP-PCP (β,γ-methyleneadenosine 5'-triphosphate) and SP20. The product structures together with the pseudo-Michaelis complex provide snapshots of different stages of the phosphorylation reaction. Comparison of these structures reveals conformational, coordination, and hydrogen bonding changes that might occur during the reaction and shed new light on its mechanism, roles of metals, and active site residues.
Figures




References
-
- Adams J. A. (2001) Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 101, 2271–2290. - PubMed
-
- Johnson D. A.; Akamine P.; Radzio-Andzelm E.; Madhusudan M.; Taylor S. S. (2001) Dynamics of cAMP-dependent protein kinase. Chem. Rev. 101, 2243–2270. - PubMed
-
- Taylor S. S., Buechler J. A., and Knighton D. R. (1990) Peptides and protein phosphorylation (Kemp B. E., Ed.) pp 1–41, CRC Press, Boca Raton, FL.
-
- Shaffer J.; Adams J. A. (1999) An ATP-linked structural change in protein kinase A precedes phosphoryl transfer under physiological magnesium concentrations. Biochemistry 38, 5572–5581. - PubMed
-
- Lew J.; Taylor S. S.; Adams J. A. (1997) Identification of a partially rate-determining step in the catalytic mechanism of cAMP-dependent protein kinase: A transient kinetic study using stopped-flow fluorescence spectroscopy. Biochemistry 36, 6717–6724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

