T-cell immunoglobulin and mucin domain 1 deficiency eliminates airway hyperreactivity triggered by the recognition of airway cell death
- PMID: 23672783
- PMCID: PMC3732546
- DOI: 10.1016/j.jaci.2013.03.025
T-cell immunoglobulin and mucin domain 1 deficiency eliminates airway hyperreactivity triggered by the recognition of airway cell death
Abstract
Background: Studies of asthma have been limited by a poor understanding of how nonallergic environmental exposures, such as air pollution and infection, are translated in the lung into inflammation and wheezing.
Objective: Our goal was to understand the mechanism of nonallergic asthma that leads to airway hyperreactivity (AHR), a cardinal feature of asthma independent of adaptive immunity.
Method: We examined mouse models of experimental asthma in which AHR was induced by respiratory syncytial virus infection or ozone exposure using mice deficient in T-cell immunoglobulin and mucin domain 1 (TIM1/HAVCR1), an important asthma susceptibility gene.
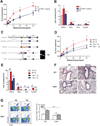
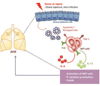
Results: TIM1(-/-) mice did not have airways disease when infected with RSV or when repeatedly exposed to ozone, a major component of air pollution. On the other hand, the TIM1(-/-) mice had allergen-induced experimental asthma, as previously shown. The RSV- and ozone-induced pathways were blocked by treatment with caspase inhibitors, indicating an absolute requirement for programmed cell death and apoptosis. TIM-1-expressing, but not TIM-1-deficient, natural killer T cells responded to apoptotic airway epithelial cells by secreting cytokines, which mediated the development of AHR.
Conclusion: We defined a novel pathway in which TIM-1, a receptor for phosphatidylserine expressed by apoptotic cells, drives the development of asthma by sensing and responding to injured and apoptotic airway epithelial cells.
Keywords: AHR; Airway hyperreactivity; BAL; Bronchoalveolar lavage; NKT; Natural killer T; OVA; Ovalbumin; Phosphatidylserine; PtdSer; RSV; Respiratory syncytial virus; T-cell immunoglobulin and mucin domain; TIM; TIM-1; TUNEL; Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling; WT; Wild-type; apoptosis; asthma; natural killer T; α-GalCer; α-Galactosylceramide.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

