The connection between inner membrane topology and mitochondrial function
- PMID: 23672826
- PMCID: PMC4219563
- DOI: 10.1016/j.yjmcc.2013.05.001
The connection between inner membrane topology and mitochondrial function
Abstract
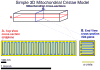
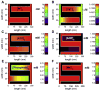
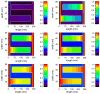
The mitochondrial inner membrane has a complex and dynamic structure that plays an important role in the function of this organelle. The internal compartments called cristae are created by processes that are just beginning to be understood. Crista size and morphology influence the internal diffusion of solutes and the surface area of the inner membrane, which is home to critical membrane proteins including ATP synthase and electron transport chain complexes; metabolite and ion transporters including the adenine nucleotide translocase, the calcium uniporter (MCU), and the sodium/calcium exchanger (NCLX); and many more. Here we provide a brief overview of what is known about crista structure and formation, and discuss mitochondrial function in the context of that structure. We also suggest that mathematical modeling of mitochondria that incorporates accurate information about the organelle's internal architecture can lead to a better understanding of its diverse functions. This article is part of a Special Issue entitled 'Calcium Signalling in Heart'.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None declared.
Figures
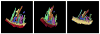


References
-
- Munn EA. The structure of mitochondria. London: Academic Press; 1974.
-
- Mitchell P. Keilin’s respiratory chain concept and its chemiosmotic consequences. Science. 1979;206:1148–59. - PubMed
-
- Williams RJ. Mitochondria and chloroplasts: localized and delocalized bioenergetic transduction. Trends Biochem Sci. 2000;25:479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

