Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production
- PMID: 23673310
- PMCID: PMC3706576
- DOI: 10.1016/j.neurobiolaging.2013.04.015
Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production
Abstract
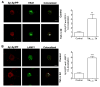
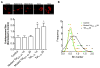
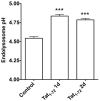
The increased life expectancy of people living with HIV-1/AIDS is accompanied by increased prevalence of HIV-1-associated neurocognitive disorder. As well, these individuals are increasingly experiencing Alzheimer's disease (AD)-like neurocognitive problems and neuropathological features such as increased deposition of amyloid beta (Aβ) protein. Findings that Aβ production occurs largely in endolysosomes, that HIV-1 transactivator protein (Tat) disrupts endolysosome function-an early pathological feature of AD-and that HIV-1 Tat can increase Aβ levels prompted us to test the hypothesis that endolysosome dysfunction is associated with HIV-1 Tat-induced increases in neuronal Aβ generation. Using primary cultured rat hippocampal neurons, we found that treatment with HIV-1 Tat caused such morphological changes as enlargement of endolysosomes identified with LysoTracker dye and such functional changes as elevated endolysosome pH measured ratiometrically with LysoSensor dye. The HIV-1 Tat-induced changes in endolysosome function preceded temporally HIV-1 Tat-induced increases in Aβ generation measured using enzyme-linked immunosorbent assay. In addition, we demonstrated that HIV-1 Tat increased endolysosome accumulation of Aβ precursor protein and Aβ identified using immunostaining with 4G8 antibodies. Furthermore, we demonstrated that treatment of neurons with HIV-1 Tat increased endolysosome accumulation of beta amyloid-converting enzyme, the rate-limiting enzymatic step for Aβ production, and enhanced beta amyloid-converting enzyme activity. Together, our findings suggest that HIV-1 Tat increases neuronal Aβ generation and thereby contributes to the development of AD-like pathology in HIV-1-infected individuals by disturbing endolysosome structure and function.
Keywords: Amyloid beta; BACE-1; Endolysosome; HIV-1 Tat; pH.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no current or potential conflicts of interest to report.
The authors have no current or potential conflicts of interest to report.
Figures
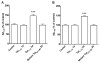

Similar articles
-
Endolysosome involvement in LDL cholesterol-induced Alzheimer's disease-like pathology in primary cultured neurons.Life Sci. 2012 Dec 10;91(23-24):1159-68. doi: 10.1016/j.lfs.2012.04.039. Epub 2012 May 11. Life Sci. 2012. PMID: 22580286 Free PMC article.
-
Antiretroviral Drugs Promote Amyloidogenesis by De-Acidifying Endolysosomes.J Neuroimmune Pharmacol. 2021 Mar;16(1):159-168. doi: 10.1007/s11481-019-09862-1. Epub 2019 Jul 23. J Neuroimmune Pharmacol. 2021. PMID: 31338753 Free PMC article.
-
Caffeine Blocks HIV-1 Tat-Induced Amyloid Beta Production and Tau Phosphorylation.J Neuroimmune Pharmacol. 2017 Mar;12(1):163-170. doi: 10.1007/s11481-016-9707-4. Epub 2016 Sep 15. J Neuroimmune Pharmacol. 2017. PMID: 27629410 Free PMC article.
-
Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer's disease?J Neurovirol. 2019 Oct;25(5):634-647. doi: 10.1007/s13365-019-00732-3. Epub 2019 Mar 13. J Neurovirol. 2019. PMID: 30868421 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
17⍺-Estradiol Protects against HIV-1 Tat-Induced Endolysosome Dysfunction and Dendritic Impairments in Neurons.Cells. 2023 Mar 6;12(5):813. doi: 10.3390/cells12050813. Cells. 2023. PMID: 36899948 Free PMC article.
-
A biological perspective of CSF lipids as surrogate markers for cognitive status in HIV.J Neuroimmune Pharmacol. 2013 Dec;8(5):1136-46. doi: 10.1007/s11481-013-9506-0. Epub 2013 Nov 8. J Neuroimmune Pharmacol. 2013. PMID: 24203462 Free PMC article. Review.
-
Dimethyl Fumarate Prevents HIV-Induced Lysosomal Dysfunction and Cathepsin B Release from Macrophages.J Neuroimmune Pharmacol. 2018 Sep;13(3):345-354. doi: 10.1007/s11481-018-9794-5. Epub 2018 Jul 9. J Neuroimmune Pharmacol. 2018. PMID: 29987592 Free PMC article.
-
HIV-Associated Insults Modulate ADAM10 and Its Regulator Sirtuin1 in an NMDA Receptor-Dependent Manner.Cells. 2022 Sep 22;11(19):2962. doi: 10.3390/cells11192962. Cells. 2022. PMID: 36230925 Free PMC article.
-
Lysosomal Stress Response (LSR): Physiological Importance and Pathological Relevance.J Neuroimmune Pharmacol. 2021 Jun;16(2):219-237. doi: 10.1007/s11481-021-09990-7. Epub 2021 Mar 22. J Neuroimmune Pharmacol. 2021. PMID: 33751445 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

