Coordinated activation of the Rac-GAP β2-chimaerin by an atypical proline-rich domain and diacylglycerol
- PMID: 23673634
- PMCID: PMC3700536
- DOI: 10.1038/ncomms2834
Coordinated activation of the Rac-GAP β2-chimaerin by an atypical proline-rich domain and diacylglycerol
Abstract
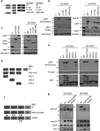
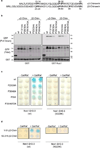
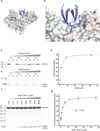
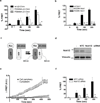
Chimaerins, a family of GTPase activating proteins for the small G-protein Rac, have been implicated in development, neuritogenesis and cancer. These Rac-GTPase activating proteins are regulated by the lipid second messenger diacylglycerol generated by tyrosine kinases such as the epidermal growth factor receptor. Here we identify an atypical proline-rich motif in chimaerins that binds to the adaptor protein Nck1. Unlike most Nck1 partners, chimaerins bind to the third SH3 domain of Nck1. This association is mediated by electrostatic interactions of basic residues within the Pro-rich motif with acidic clusters in the SH3 domain. Epidermal growth factor promotes the binding of β2-chimaerin to Nck1 in the cell periphery in a diacylglycerol-dependent manner. Moreover, β2-chimaerin translocation to the plasma membrane and its peripheral association with Rac1 requires Nck1. Our studies underscore a coordinated mechanism for β2-chimaerin activation that involves lipid interactions via the C1 domain and protein-protein interactions via the N-terminal proline-rich region.
Figures



References
-
- Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, et al. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron. 2007;55:756–767. - PubMed
-
- Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–778. - PubMed
-
- Suliman SG, Stanik J, McCulloch LJ, Wilson N, Edghill EL, Misovicova N, et al. Severe insulin resistance and intrauterine growth deficiency associated with haploinsufficiency for INSR and CHN2: new insights into synergistic pathways involved in growth and metabolism. Diabetes. 2009;58:2954–2961. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

