Regulation of the yeast triacylglycerol lipase TGl3p by formation of nonpolar lipids
- PMID: 23673660
- PMCID: PMC3707694
- DOI: 10.1074/jbc.M113.459610
Regulation of the yeast triacylglycerol lipase TGl3p by formation of nonpolar lipids
Abstract
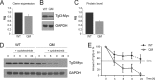
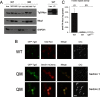
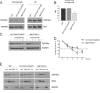
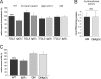
Tgl3p, the major triacylglycerol lipase of the yeast Saccharomyces cerevisiae, is a component of lipid droplets but is also present in the endoplasmic reticulum in a minor amount. Recently, it was shown that this enzyme can also serve as a lysophospholipid acyltransferase (Rajakumari, S., and Daum, G. (2010) Mol. Biol. Cell 21, 501-510). Here, we describe the effects of the presence/absence of triacylglycerols and lipid droplets on the functionality of Tgl3p. In a dga1Δlro1Δare1Δare2Δ quadruple mutant lacking all four triacylglycerol- and steryl ester-synthesizing acyltransferases and consequently the lipid droplets, the gene expression of TGL3 was only slightly altered. In contrast, protein level and stability of Tgl3p were markedly reduced in the absence of lipid droplets. Under these conditions, the enzyme was localized to the endoplasmic reticulum. Even the lack of the substrate, triacylglycerol, affected stability and localization of Tgl3p to some extent. Interestingly, Tgl3p present in the endoplasmic reticulum seems to lack lipolytic as well as acyltransferase activity as shown by enzymatic analysis and lipid profiling. Thus, we propose that the activity of Tgl3p is restricted to lipid droplets, whereas the endoplasmic reticulum may serve as a parking lot for this enzyme.
Keywords: Acyltransferase; Endoplasmic Reticulum (ER); Lipase; Lipid Droplets; Triacylglycerol; Yeast.
Figures

References
-
- Zweytick D., Athenstaedt K., Daum G. (2000) Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta 1469, 101–120 - PubMed
-
- Leber R., Zinser E., Zellnig G., Paltauf F., Daum G. (1994) Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10, 1421–1428 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

