11CO2 fixation: a renaissance in PET radiochemistry
- PMID: 23673726
- PMCID: PMC5604310
- DOI: 10.1039/c3cc42236d
11CO2 fixation: a renaissance in PET radiochemistry
Abstract
Carbon-11 labelled carbon dioxide is the cyclotron-generated feedstock reagent for most positron emission tomography (PET) tracers using this radionuclide. Most carbon-11 labels, however, are installed using derivative reagents generated from [(11)C]CO2. In recent years, [(11)C]CO2 has seen a revival in applications for the direct incorporation of carbon-11 into functional groups such as ureas, carbamates, oxazolidinones, carboxylic acids, esters, and amides. This review summarizes classical [(11)C]CO2 fixation strategies using organometallic reagents and then focuses on newly developed methods that employ strong organic bases to reversibly capture [(11)C]CO2 into solution, thereby enabling highly functionalized labelled compounds to be prepared. Labelled compounds and radiopharmaceuticals that have been translated to the clinic are highlighted.
Figures



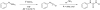
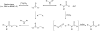

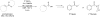
References
-
- Ametamey SM, Horner M, Schubiger PA. Chem Rev. 2008;108:1501. - PubMed
-
- Ferrieri RA. In: Handbook of Radiopharmaceuticals. Welch MJ, Redvanly CS, editors. John Wiley & Sons, Ltd.; Chichester: 2003. pp. 229–282.
-
- Bolton R. J Labelled Compd Radiopharm. 2001;44:701.
-
- Allard M, Fouquet E, James D, Szlosek-Pinaud M. Curr Med Chem. 2008;15:235. - PubMed
-
- Roeda D, Crouzel C, van Zanten B. Radiochem Radioanal Lett. 1978;33:175.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

