The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study
- PMID: 23674289
- PMCID: PMC3826053
- DOI: 10.1002/mus.23905
The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study
Abstract
Introduction: An international clinical trial enrolled 174 ambulatory males ≥5 years old with nonsense mutation Duchenne muscular dystrophy (nmDMD). Pretreatment data provide insight into reliability, concurrent validity, and minimal clinically important differences (MCIDs) of the 6-minute walk test (6MWT) and other endpoints.
Methods: Screening and baseline evaluations included the 6-minute walk distance (6MWD), timed function tests (TFTs), quantitative strength by myometry, the PedsQL, heart rate-determined energy expenditure index, and other exploratory endpoints.
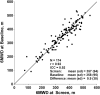
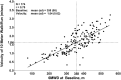
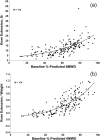
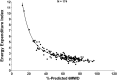
Results: The 6MWT proved feasible and reliable in a multicenter context. Concurrent validity with other endpoints was excellent. The MCID for 6MWD was 28.5 and 31.7 meters based on 2 statistical distribution methods.
Conclusions: The ratio of MCID to baseline mean is lower for 6MWD than for other endpoints. The 6MWD is an optimal primary endpoint for Duchenne muscular dystrophy (DMD) clinical trials that are focused therapeutically on preservation of ambulation and slowing of disease progression.
Keywords: 6-minute walk test; Duchenne muscular dystrophy; PedsQL; ambulation; energy expenditure index; muscular dystrophy; myometry; natural history; timed function test.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
Comment in
-
Beyond the gowers sign: measuring outcomes in Duchenne muscular dystrophy.Muscle Nerve. 2013 Sep;48(3):315-7. doi: 10.1002/mus.23984. Muscle Nerve. 2013. PMID: 24038058 No abstract available.
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchennemuscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. - PubMed
-
- Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years—four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–1122. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

