Proteome-wide discovery of mislocated proteins in cancer
- PMID: 23674306
- PMCID: PMC3730102
- DOI: 10.1101/gr.155499.113
Proteome-wide discovery of mislocated proteins in cancer
Abstract
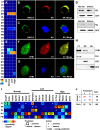
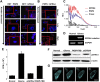
Several studies have sought systematically to identify protein subcellular locations, but an even larger task is to map which of these proteins conditionally relocates in disease (the mislocalizome). Here, we report an integrative computational framework for mapping conditional location and mislocation of proteins on a proteome-wide scale, called a conditional location predictor (CoLP). Using CoLP, we mapped the locations of over 10,000 proteins in normal human brain and in glioma. The prediction showed 0.9 accuracy using 100 location tests of 20 randomly selected proteins. Of the 10,000 proteins, over 150 have a strong likelihood of mislocation under glioma, which is striking considering that few mislocation events have been identified in this disease previously. Using immunofluorescence and Western blotting in both primary cells and tissues, we successfully experimentally confirmed 15 mislocations. The most common type of mislocation occurs between the endoplasmic reticulum and the nucleus; for example, for RNF138, TLX3, and NFRKB. In particular, we found that the gene for the mislocating protein GFRA4 had a nonsynonymous point mutation in exon 2. Moreover, redirection of GFRA4 to its normal location, the plasma membrane, led to marked reductions in phospho-STAT3 and proliferation of glioma cells. This framework has the potential to track changes in protein location in many human diseases.
Figures





Similar articles
-
Role of STAT3 in Genesis and Progression of Human Malignant Gliomas.Mol Neurobiol. 2017 Oct;54(8):5780-5797. doi: 10.1007/s12035-016-0103-0. Epub 2016 Sep 22. Mol Neurobiol. 2017. PMID: 27660268 Review.
-
Paracrine regulation of glioma cells invasion by astrocytes is mediated by glial-derived neurotrophic factor.Int J Cancer. 2015 Sep 1;137(5):1012-20. doi: 10.1002/ijc.29380. Epub 2014 Dec 13. Int J Cancer. 2015. PMID: 25487790
-
GOLPH3 promotes glioma progression via facilitating JAK2-STAT3 pathway activation.J Neurooncol. 2018 Sep;139(2):269-279. doi: 10.1007/s11060-018-2884-7. Epub 2018 Apr 30. J Neurooncol. 2018. PMID: 29713848
-
Doublecortin undergo nucleocytoplasmic transport via the RanGTPase signaling to promote glioma progression.Cell Commun Signal. 2020 Feb 12;18(1):24. doi: 10.1186/s12964-019-0485-5. Cell Commun Signal. 2020. PMID: 32050972 Free PMC article.
-
Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas.Acta Neuropathol. 2000 Feb;99(2):131-7. doi: 10.1007/pl00007416. Acta Neuropathol. 2000. PMID: 10672319
Cited by
-
Histological Analysis, Bioinformatics Profile, and Expression of Methylenetetrahydrofolate Reductase (MTHFR) in Bovine Testes.Animals (Basel). 2020 Sep 23;10(10):1731. doi: 10.3390/ani10101731. Animals (Basel). 2020. PMID: 32977696 Free PMC article.
-
RNF138-mediated ubiquitination of rpS3 is required for resistance of glioblastoma cells to radiation-induced apoptosis.Exp Mol Med. 2018 Jan 26;50(1):e434. doi: 10.1038/emm.2017.247. Exp Mol Med. 2018. PMID: 29371697 Free PMC article.
-
An integrated transcriptomic and computational analysis for biomarker identification in human glioma.Tumour Biol. 2016 Jun;37(6):7185-92. doi: 10.1007/s13277-015-4585-6. Epub 2015 Dec 14. Tumour Biol. 2016. PMID: 26663173
-
NF-κB and STAT3 in glioblastoma: therapeutic targets coming of age.Expert Rev Neurother. 2014 Nov;14(11):1293-306. doi: 10.1586/14737175.2014.964211. Epub 2014 Sep 29. Expert Rev Neurother. 2014. PMID: 25262780 Free PMC article. Review.
-
Role of STAT3 in Genesis and Progression of Human Malignant Gliomas.Mol Neurobiol. 2017 Oct;54(8):5780-5797. doi: 10.1007/s12035-016-0103-0. Epub 2016 Sep 22. Mol Neurobiol. 2017. PMID: 27660268 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
