Adoptive regulatory T-cell therapy protects against cerebral ischemia
- PMID: 23674483
- PMCID: PMC3748165
- DOI: 10.1002/ana.23815
Adoptive regulatory T-cell therapy protects against cerebral ischemia
Abstract
Objective: Recent evidence suggests that functional deficiency in regulatory T cells (Tregs), an innate immunomodulator, exacerbates brain damage after cerebral ischemia. We therefore evaluated the effect of Treg transfer in rodent models of ischemic stroke and further investigated the mechanism underlying Treg-afforded neuroprotection.
Methods: We examined the therapeutic potential of Tregs and the mechanisms of neuroprotection in vivo in 2 rodent models of ischemic stroke and in vitro in Treg-neutrophil cocultures using a combined approach including cell-specific depletion, gene knockout mice, and bone marrow chimeras.
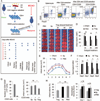
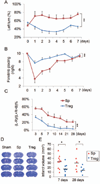
Results: Systemic administration of purified Tregs at 2, 6, or even 24 hours after middle cerebral artery occlusion resulted in a marked reduction of brain infarct and prolonged improvement of neurological functions lasting out to 4 weeks. Treg-afforded neuroprotection was accompanied by attenuated blood-brain barrier (BBB) disruption during early stages of ischemia, decreased cerebral inflammation, and reduced infiltration of peripheral inflammatory cells into the lesioned brain. Surprisingly, Tregs exerted early neuroprotection without penetrating into the brain parenchyma or inhibiting the activation of residential microglia. Rather, both in vivo and in vitro studies demonstrated that Tregs suppressed peripheral neutrophil-derived matrix metallopeptidase-9 production, thus preventing proteolytic damage of the BBB. In addition to its potent central neuroprotection, Treg treatment was shown to ameliorate poststroke lymphopenia, suggesting a beneficial effect on immune status.
Interpretation: Our study suggests that Treg adoptive therapy is a novel and potent cell-based therapy targeting poststroke inflammatory dysregulation and neurovascular disruption.
Copyright © 2012 American Neurological Association.
Figures

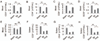




References
-
- Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007 Apr;61(4):352–362. - PubMed
-
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006 Apr;37(4):1087–1093. - PubMed
-
- Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. American journal of physiology. 2005 Aug;289(2):H558–H568. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

