In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages
- PMID: 23674673
- PMCID: PMC3670364
- DOI: 10.1073/pnas.1303858110
In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages
Abstract
CD8(+) T cells are specialized cells of the adaptive immune system capable of finding and eliminating pathogen-infected cells. To date it has not been possible to observe the destruction of any pathogen by CD8(+) T cells in vivo. Here we demonstrate a technique for imaging the killing of liver-stage malaria parasites by CD8(+) T cells bearing a transgenic T cell receptor specific for a parasite epitope. We report several features that have not been described by in vitro analysis of the process, chiefly the formation of large clusters of effector CD8(+) T cells around infected hepatocytes. The formation of clusters requires antigen-specific CD8(+) T cells and signaling by G protein-coupled receptors, although CD8(+) T cells of unrelated specificity are also recruited to clusters. By combining mathematical modeling and data analysis, we suggest that formation of clusters is mainly driven by enhanced recruitment of T cells into larger clusters. We further show various death phenotypes of the parasite, which typically follow prolonged interactions between infected hepatocytes and CD8(+) T cells. These findings stress the need for intravital imaging for dissecting the fine mechanisms of pathogen recognition and killing by CD8(+) T cells.
Keywords: Plasmodium; immunity; lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
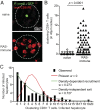


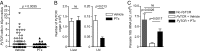
References
-
- Romero P, et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341(6240):323–326. - PubMed
-
- Trimnell A, et al. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J Immunol. 2009;183(9):5870–5878. - PubMed
-
- Morrot A, Zavala F. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol Rev. 2004;201:291–303. - PubMed
-
- Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25(1):129–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

