Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs
- PMID: 23674677
- PMCID: PMC3670320
- DOI: 10.1073/pnas.1217206110
Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs
Abstract
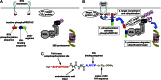
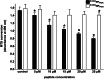
Posttranslational knockdown of a specific protein is an attractive approach for examining its function within a system. Here we introduce phospho-dependent proteolysis targeting chimeras (phosphoPROTACs), a method to couple the conditional degradation of targeted proteins to the activation state of particular kinase-signaling pathways. We generated two phosphoPROTACs that couple the tyrosine phosphorylation sequences of either the nerve growth factor receptor, TrkA (tropomyosin receptor kinase A), or the neuregulin receptor, ErbB3 (erythroblastosis oncogene B3), with a peptide ligand for the E3 ubiquitin ligase von Hippel Lindau protein. These phosphoPROTACs recruit either the neurotrophic signaling effector fibroblast growth factor receptor substrate 2α or the survival-promoting phosphatidylinositol-3-kinase, respectively, to be ubiquitinated and degraded upon activation of specific receptor tyrosine kinases and phosphorylation of the phosphoPROTACs. We demonstrate the ability of these phosphoPROTACs to suppress the short- and long-term effects of their respective activating receptor tyrosine kinase pathways both in vitro and in vivo. In addition, we show that activation of phosphoPROTACs is entirely dependent on their kinase-mediated phosphorylation, as phenylalanine-containing null variants are inactive. Furthermore, stimulation of unrelated growth factor receptors does not induce target protein knockdown. Although comparable in efficiency to RNAi, this approach has the added advantage of providing a degree of temporal and dosing control as well as cell-type selectivity unavailable using nucleic acid-based strategies. By varying the autophosphorylation sequence of a phosphoPROTAC, it is conceivable that other receptor tyrosine kinase/effector pairings could be similarly exploited to achieve other biological effects.
Keywords: SH2 domain; protein degradation; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

