Chiral drugs: an overview
Abstract
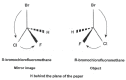
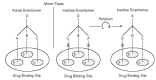
About more than half of the drugs currently in use are chiral compounds and near 90% of the last ones are marketed as racemates consisting of an equimolar mixture of two enantiomers. Although they have the same chemical structure, most isomers of chiral drugs exhibit marked differences in biological activities such as pharmacology, toxicology, pharmacokinetics, metabolism etc. Some mechanisms of these properties are also explained. Therefore, it is important to promote the chiral separation and analysis of racemic drugs in pharmaceutical industry as well as in clinic in order to eliminate the unwanted isomer from the preparation and to find an optimal treatment and a right therapeutic control for the patient. In this article, we review the nomenclature, pharmacology, toxicology, pharmacokinetics, metabolism etc of some usual chiral drugs as well as their mechanisms. Different techniques used for the chiral separation in pharmaceutical industry as well as in clinical analyses are also examined.
Keywords: analysis; chiral drugs; chiral separation; chiral terms; enantioselective antibodies; metabolism; pharmacokinetics; pharmacology; toxicology.
Figures
References
-
- Challener CA. In: Chiral drugs. 1st. Aldershot (England): Ashgate Publisher; 2001. Overview of chirality; pp. 3–14.
-
- Drayer DE. The early history of stereochemistry. In: Wainer IW, editor. Drug stereochemistry. Analytical methods and pharmacology. 2nd. New York: Marcel Dekker Publisher; 1993. pp. 1–24.
-
- Rentsch KM. The importance of stereoselective determination of drugs in the clinical laboratory. Journal of Biochemical and Biophysical Methods. 2002;54(1-3):1–9. - PubMed
-
- Walther W, Netscher T. Design and development of chiral reagents for the chromatographic determination of chiral alcohols. Chirality. 1996;8:397–401.
-
- Katzung BG. In: Basic and clinical pharmacology. 9th. New York: Lange Medical Books/McGraw Hill; 2004. The nature of drugs; pp. 3–5.
LinkOut - more resources
Full Text Sources
Other Literature Sources
