Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances
- PMID: 23674985
- PMCID: PMC3614608
Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances
Abstract
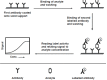
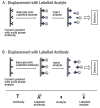
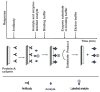
Immunoassays are bioanalytical methods in which the quantitation of the analyte depends on the reaction of an antigen (analyte) and an antibody. Immunoassays have been widely used in many important areas of pharmaceutical analysis such as diagnosis of diseases, therapeutic drug monitoring, clinical pharmacokinetic and bioequivalence studies in drug discovery and pharmaceutical industries. The importance and widespread of immunoassay methods in pharmaceutical analysis are attributed to their inherent specificity, high-throughput, and high sensitivity for the analysis of wide range of analytes in biological samples. Recently, marked improvements were achieved in the field of immunoassay development for the purposes of pharmaceutical analysis. These improvements involved the preparation of the unique immunoanalytical reagents, analysis of new categories of compounds, methodology, and instrumentation. The basic methodologies and recent advances in immunoassay methods applied in different fields of pharmaceutical analysis have been reviewed.
Keywords: antibodies; drug discovery; immunoassay; pharmaceutical analysis; pharmaceutical industry.
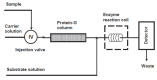
Figures




References
-
- Kellner R, Mermet JM, Otto M, Widmer HM. Analytical Chemistry. New York: Wiley-VCH; 1998. pp. 405–429.
-
- Findlay JW, Smith WC, Lee JW, Nordblom GD, et al. J. Pharm. Biomed. Anal. 2000;21:1249–1273. - PubMed
-
- Chuanlai X, Cifang P, Kai H, Zhengyu J, et al. Luminescence. 2006;21:126–128. - PubMed
-
- Samsonova ZhV, Shchelokova OS, Ivanova NL, Rubtsova MU, et al. Prikl. Biokhim. Mikrobiol. 2005;41:668–675. - PubMed
-
- Lachenmeier K, Musshoff F, Madea B. Forensic. Sci. Intl. 2006;159:189–199. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
