Spectrophotometric determination of labetalol and lercanidipine in pure form and in pharmaceutical preparations using ferric-1,10-phenanthroline
- PMID: 23675146
- PMCID: PMC3614790
Spectrophotometric determination of labetalol and lercanidipine in pure form and in pharmaceutical preparations using ferric-1,10-phenanthroline
Abstract
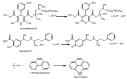
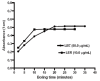
A simple and sensitive spectrophotometric method was developed for the determination of labetalol HCl (LBT) and lercanidipine HCl (LER) in pure form and in dosage forms. The method was based upon oxidation of the LBT and LER with Fe(+3) and the estimation of the produced Fe(+2) with 1,10-phenanthroline. The absorbance of the tris(1,10-phenanthroline) Fe(+2) complex was measured at 510 nm. Reaction conditions were optimized to obtain colored complex of higher sensitivity and longer stability. The absorbance concentration plots were rectilinear over the concentration rang of 5-90 and 1-20 μg/mL with lower detection limits of 0.74 and 0.01 μg/mL and quantification limits of 2.26 and 0.02 μg/mL for LBT and LER, respectively. The developed method was successfully applied for the determination of LBT and LER in bulk drugs and dosage forms. The common excipients and additives did not interfere in their determinations. There was no significant difference between the results obtained by the proposed and the reference methods regarding Student t-test and the variance ratio F-test.
Keywords: Ferric-1,10-Phenanthroline; labetalol; lercanidipine; spectrophotometry.
Figures


Similar articles
-
Spectrophotometric Determination of the Antidepressants Sertraline and Paroxetine HCl using 2,4-Dinitrofluorobenzene.Int J Biomed Sci. 2010 Sep;6(3):252-9. Int J Biomed Sci. 2010. PMID: 23675200 Free PMC article.
-
Sensitive spectrophotometric methods for the determination of amoxycillin, ciprofloxacin and piroxicam in pure and pharmaceutical formulations.J Pharm Biomed Anal. 2002 Jul 31;29(5):859-64. doi: 10.1016/s0731-7085(02)00210-8. J Pharm Biomed Anal. 2002. PMID: 12093519
-
A sensitive spectrophotometric method for the determination of propranolol HCl based on oxidation bromination reactions.Drug Test Anal. 2010 Mar;2(3):122-9. doi: 10.1002/dta.103. Drug Test Anal. 2010. PMID: 20878893
-
Derivatization of labetalol hydrochloride for its spectrofluorimetric and spectrophotometric determination inhuman plasma: Application to stability study.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Feb 5;190:457-463. doi: 10.1016/j.saa.2017.09.059. Epub 2017 Sep 22. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 28961530
-
Validated spectrophotometric and spectrofluorimetric methods for determination of dapoxetine hydrochloride and dosulepin hydrochloride in their dosage forms using mercurochrome.Luminescence. 2018 Dec;33(8):1306-1313. doi: 10.1002/bio.3566. Epub 2018 Oct 30. Luminescence. 2018. PMID: 30378237 Review.
Cited by
-
A fast, stability-indicating, and validated liquid chromatography method for the purity control of lercanidipine hydrochloride in tablet dosage form.Sci Pharm. 2014 Jan 16;82(2):327-40. doi: 10.3797/scipharm.1310-10. Print 2014 Apr-Jun. Sci Pharm. 2014. PMID: 24959405 Free PMC article.
-
Development of three ecological spectroscopic methods for analysis of betrixaban either alone or in mixture with lercanidipine: greenness assessment.R Soc Open Sci. 2022 Feb 2;9(2):211457. doi: 10.1098/rsos.211457. eCollection 2022 Feb. R Soc Open Sci. 2022. PMID: 35127114 Free PMC article.
References
-
- Sweetman SC. Martindale: The Complete Drug Reference. 35th edition. London: The Pharmaceutical Press; 2007. Electronic Version.
-
- The British Pharmacopoeia. London: Her Majesty's Stationery Office; 2007. Electronic Version.
-
- Rockville, MD: US Pharmacopeial Convention; 2007. The United States Pharmacopoeia 30, the National Formulary 25. Electronic version.
-
- Nafisur R, Habibur R, Syed N, et al. J. Chinese Chem. Soci. 2007;54:185–196.
-
- Nafisur R, Manisha S, Nasrul MD, et al. Chinese J. Chem. 2005;23:1611–1617.
LinkOut - more resources
Full Text Sources
