Diethylcarbamazine and non-diethylcarbamazine related bancroftian granuloma: an immunohistochemical study of eosinophil toxic proteins
- PMID: 23675184
- PMCID: PMC3614742
Diethylcarbamazine and non-diethylcarbamazine related bancroftian granuloma: an immunohistochemical study of eosinophil toxic proteins
Abstract
It has been suggested, mostly using in vitro experiments, that defenses against parasites involve mainly activated eosinophils and their toxic proteins, such as major basic protein (MBP), eosinophil cationic protein (ECP) and eosinophil peroxidase (EPO). Eosinophil degranulation has been described around degenerating onchocercal microfilariae in patients treated with diethylcarbamazine (DEC). In bancroftian filariasis, traditional histopathologic studies have shown remarkable numbers of eosinophils in granulomatous lesions associated with both DEC-induced and spontaneous death of adult Wuchereria bancrofti parasites. No immunohistochemical study targeting eosinophil degranulation has been previously performed in these granulomas, which are found mainly within intrascrotal lymphatic vessels. This investigation was undertaken in 22 (12 DEC-treated and 10 untreated) male patients in order to determine the immunohistochemical expressions of MBP, EPO and ECP in bancofitian granulomas, using the indirect method. Stained intact esosinophils, as well as granular, extra-cellular material positive for all three proteins, were found in all granulomas. The immunohistochemical patterns were similar in both DEC-treated and untreated cases, irrespective of microfilaremia, blood eosinophilia, and granuloma age. Positive intact cells were observed mostly at the periphery of the granulomas, whereas granular material predominated in central areas around dead or degenerating parasites. These results indicate that eosinophils accumulate in the granulomas and degranulate preferentially in close proximity to degenerating or dead adult parasites. In bancroftian granulomas, influx and degranulation of eosinophils are considered a consequence of parasite death, rather than its cause.
Keywords: Wuchereria bancrofti; bancroftian filariasis; eosinophil cationic protein; eosinophil peroxidase; eosinophils; filarial granuloma; immunohistochemistry; lymphatic fiariasis; major basic protein.
Figures

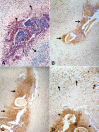
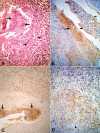

References
-
- Rothenberg ME. Eosinophilia. N. Engl. J. Med. 1998;338(22):1592. - PubMed
-
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004;113(1):30. - PubMed
-
- Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16(3):95. - PubMed
-
- Butterworth AE, Sturrock R, Houba V, et al. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256(5520):727. - PubMed
-
- Greene BM, Taylor HR, Aikawa M. Cellular killing of microfilariae of Onchocerca volvulus: eosinophil and neutrophil mediated immune serum dependent destruction. J. Immunol. 1981;127(4):1611. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
