Liquid chromatographic methods for the determination of vildagliptin in the presence of its synthetic intermediate and the simultaneous determination of pioglitazone hydrochloride and metformin hydrochloride
- PMID: 23675237
- PMCID: PMC3614836
Liquid chromatographic methods for the determination of vildagliptin in the presence of its synthetic intermediate and the simultaneous determination of pioglitazone hydrochloride and metformin hydrochloride
Abstract
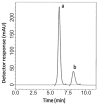
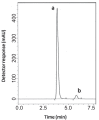
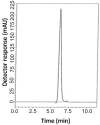
Two reversed-phase liquid chromatographic (RP-LC) methods are described for the determination of two binary mixtures of hypoglycemic agents. In the first method, vildagliptin (VDG) was determined in the presence of 3-amino-1-adamantanol (AAD), a synthetic intermediate and impurity of VDG. In the second method, pioglitazone hydrochloride (PGZ) and metformin hydrochloride (MET) were simultaneously determined in their binary mixture. Chromatographic separation in the two methods was achieved on a Symmetry(®) Waters C18 column (150 mm × 4.6 mm, 5 μm). In the first mixture, isocratic elution using a mobile phase of potassium dihydrogen phosphate buffer pH (4.6) - acetonitrile - methanol (30:50:20, v/v/v) at a flow rate of 1 mL min(-1) with UV detection at 220 nm was performed. In the second method, isocratic elution based on potassium dihydrogen phosphate buffer pH (4.6) - acetonitrile (60:40, v/v) at a flow rate of 1 mL min(-1) with UV detection at 210 nm was performed. Linearity, accuracy and precision were found to be acceptable over the concentration ranges of 5-200 μg mL(-1), 0.5-3 μg mL(-1) and 10-150 μg mL(-1) for VDG, PGZ and MET, respectively. The optimized methods were validated and proved to be specific, robust, precise and accurate for the quality control of the drugs in their pharmaceutical preparations.
Keywords: metformin hydrochloride; pioglitazone hydrochloride; reversed-phase liquid chromatography; tablets; vildagliptin.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous
