Liquid chromatographic determination of linagliptin in bulk, in plasma and in its pharmaceutical preparation
- PMID: 23675275
- PMCID: PMC3615276
Liquid chromatographic determination of linagliptin in bulk, in plasma and in its pharmaceutical preparation
Abstract
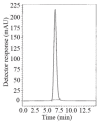
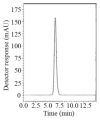
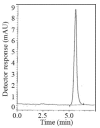
In this work, two reversed-phase liquid chromatographic (RP-LC) methods have been developed for the determination of linagliptin (LNG) based on isocratic elution using a mobile phase consisting of potassium dihydrogen phosphate buffer pH (4.6)-acetonitrile(20:80, v/v) at a flow rate of 1 mL min(-1). Two detection techniques have been applied either UV detection at 299 nm in the first method or fluorometric detection at 239 nm for excitation and 355 nm for emission in the second method. Chromatographic separation in the two methods was achieved on a Symmetry(®) cyanide column (150 mm × 4.6 mm, 5 μm). Linearity, accuracy and precision were found to be acceptable over the concentration ranges of 2.5-80 μg mL(-1) for LNG in bulk and 2.5-15 μg mL(-1) for LNG in plasma with the first method and 5-160 μg mL(-1) for LNG in bulk with the second method. The optimized methods were validated and proved to be specific, robust and accurate for the quality control of the cited drug in its pharmaceutical preparation.
Keywords: fluorometric detection; linagliptin; pharmaceutical preparation; plasma; reversed-phase liquid chromatography.
Figures
Similar articles
-
Spectrophotometric Methods for the Determination of Linagliptin in Binary Mixture with Metformin Hydrochloride and Simultaneous Determination of Linagliptin and Metformin Hydrochloride using High Performance Liquid Chromatography.Int J Biomed Sci. 2013 Mar;9(1):41-7. Int J Biomed Sci. 2013. PMID: 23675288 Free PMC article.
-
Liquid chromatographic determination of alogliptin in bulk and in its pharmaceutical preparation.Int J Biomed Sci. 2012 Sep;8(3):215-8. Int J Biomed Sci. 2012. PMID: 23675276 Free PMC article.
-
Liquid chromatographic determination of sitagliptin either alone or in ternary mixture with metformin and sitagliptin degradation product.Talanta. 2011 Jul 15;85(1):673-80. doi: 10.1016/j.talanta.2011.04.051. Epub 2011 Apr 27. Talanta. 2011. PMID: 21645757
-
Liquid chromatographic methods for the determination of vildagliptin in the presence of its synthetic intermediate and the simultaneous determination of pioglitazone hydrochloride and metformin hydrochloride.Int J Biomed Sci. 2011 Sep;7(3):201-8. Int J Biomed Sci. 2011. PMID: 23675237 Free PMC article.
-
A Validated HPLC Method for Simultaneous Determination of Perindopril Arginine, Amlodipine, and Indapamide: Application in Bulk and in Different Pharmaceutical Dosage Forms.J AOAC Int. 2017 Jul 1;100(4):992-999. doi: 10.5740/jaoacint.16-0279. Epub 2017 Feb 7. J AOAC Int. 2017. PMID: 28168948
Cited by
-
Spectrophotometric Methods for the Determination of Linagliptin in Binary Mixture with Metformin Hydrochloride and Simultaneous Determination of Linagliptin and Metformin Hydrochloride using High Performance Liquid Chromatography.Int J Biomed Sci. 2013 Mar;9(1):41-7. Int J Biomed Sci. 2013. PMID: 23675288 Free PMC article.
-
Analytical and Bioanalytical Methods for the Determination of Dipeptidyl Peptidase-4 Inhibitors in Various Matrices: A Comprehensive Review.Curr Diabetes Rev. 2025;21(5):e030524229629. doi: 10.2174/0115733998288292240409060854. Curr Diabetes Rev. 2025. PMID: 38706366 Review.
-
Biological Safety Studies and Simultaneous Determination of Linagliptin and Synthetic Impurities by LC-PDA.J Anal Methods Chem. 2019 Mar 3;2019:7534609. doi: 10.1155/2019/7534609. eCollection 2019. J Anal Methods Chem. 2019. PMID: 30944754 Free PMC article.
-
Improved Chromatographic Separation of Sitagliptin Phosphate and Metformin Hydrochloride.Int J Biomed Sci. 2015 Dec;11(4):190-4. Int J Biomed Sci. 2015. PMID: 26759536 Free PMC article.
References
-
- Kirby M, Yu D, Conor S, et al. Clinical Sci. 2010;118:31. - PubMed
-
- Sekaran B, Rani P. Int. J. Pharm. Pharm. Sci. 2010;2:4.
-
- Nirogi R, Kandikere V, Mudigonda K, et al. Biomed. Chromatogr. 2008;22:214. - PubMed
-
- Salsali A, Pratley R. Nat. Rev. Endocrin. 2007;3:450. - PubMed
-
- Mello S, Gregório B, Fernando S, et al. Clin. Sci. 2010;119:239. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
