Role of DNA Repair Pathways in Response to Zidovudine-induced DNA Damage in Immortalized Human Liver THLE2 Cells
- PMID: 23675285
- PMCID: PMC3644411
Role of DNA Repair Pathways in Response to Zidovudine-induced DNA Damage in Immortalized Human Liver THLE2 Cells
Abstract
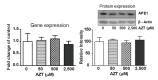
The nucleoside reverse transcriptase inhibitor zidovudine (3'-azido-3'-dexoythymidine, AZT) can be incorporated into DNA and cause DNA damage. Previously, we determined that the human hepatocellular carcinoma HepG2 cells are more susceptible to AZT-induced toxicities than the immortalized normal human liver THLE2 cells and the nucleotide excision repair (NER) pathway plays an essential role in the response to AZT-induced DNA damage. We have now investigated if the effects of AZT treatment on the expression levels of genes related to DNA damage and repair pathways contribute to the differences in sensitivity to AZT treatment between HepG2 cells and THLE2 cells. Of total 84 genes related to DNA damage and repair, two, five, and six genes were up-regulated more than 1.5-fold at 50, 500, and 2,500 µM AZT groups compared with that of control THLE2 cells. Seven genes showed a decreased expression of more than 1.5-fold following the 2,500 µM AZT treatment. Two-sided multivariate analysis of variance indicated that the change in expression of genes involved in apoptosis, cell cycle, and DNA repair pathways was significant only at 2,500 µM AZT. Statistically significant dose-related increases were identified in XPC gene expression and GTF2H1 protein level after the AZT treatments, which implicated the NER pathway in response to the DNA damage induced by AZT. In contrast, AZT treatment did not alter significantly the expression of the APE1 gene or the levels of APE1 protein. These results indicate that the NER repair pathway is involved in AZT-induced DNA damage response in immortalized human hepatic THLE2 cells.
Keywords: DNA damage and repair; THLE2 cells; nucleotide excision repair; zidovudine.
Figures


References
-
- Walker VE, Poirier MC. Special issue on health risks of perinatal exposure to nucleoside reverse transcriptase inhibitors. Environ. Mol. Mutagen. 2007;48:159–165. - PubMed
-
- Olivero OA, Beland FA, Fullerton NF, et al. Vaginal epithelial DNA damage and expression of preneoplastic markers in mice during chronic dosing with tumorigenic levels of 3’-azido-2’,3’-dideoxythymidine. Cancer Res. 1994;54:6235–6242. - PubMed
-
- Ayers KM, Clive D, Tucker WE, et al. Nonclinical toxicology studies with zidovudine: genetic toxicity tests and carcinogenicity bioassays in mice and rats. Fundam. Appl. Toxicol. 1996;32:148–158. - PubMed
-
- NTP. NTP technical report on the toxicology and carcinogenesis studies of AZT (CAS No. 30516-87-1) and AZT/α-interferon A/D B6C3F1 mice (gavage studies) Natl. Toxicol. Program Tech. Rep. Ser. 1999;469:1–361. - PubMed
-
- Olivero OA, Anderson LM, Diwan BA, et al. Transplacental effects of 3’-azido-2’,3’-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J. Natl. Cancer Inst. 1997;89:1602–1608. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
