NOD2-mediated suppression of CD55 on neutrophils enhances C5a generation during polymicrobial sepsis
- PMID: 23675299
- PMCID: PMC3649968
- DOI: 10.1371/journal.ppat.1003351
NOD2-mediated suppression of CD55 on neutrophils enhances C5a generation during polymicrobial sepsis
Abstract
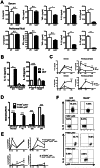
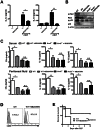
Nucleotide-binding oligomerization domain (NOD) 2 is a cytosolic protein that plays a defensive role in bacterial infection by sensing peptidoglycans. C5a, which has harmful effects in sepsis, interacts with innate proteins. However, whether NOD2 regulates C5a generation during sepsis remains to be determined. To address this issue, cecal ligation & puncture (CLP)-induced sepsis was compared in wild type and Nod2-/- mice. Nod2-/- mice showed lower levels of C5a, IL-10, and IL-1β in serum and peritoneum, but higher survival rate during CLP-induced sepsis compared to wild type mice. Injection of recombinant C5a decreased survival rates of Nod2-/- mice rate during sepsis, whereas it did not alter those in wild type mice. These findings suggest a novel provocative role for NOD2 in sepsis, in contrast to its protective role during bacterial infection. Furthermore, we found that NOD2-mediated IL-10 production by neutrophils enhanced C5a generation by suppressing CD55 expression on neutrophils in IL-1β-dependent and/or IL-1β-independent manners, thereby aggravating CLP-induced sepsis. SB203580, a receptor-interacting protein 2 (RIP2) inhibitor downstream of NOD2, reduced C5a generation by enhancing CD55 expression on neutrophils, resulting in attenuation of polymicrobial sepsis. Therefore, we propose a novel NOD2-mediated complement cascade regulatory pathway in sepsis, which may be a useful therapeutic target.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365: 63–78. - PubMed
-
- Russell JA (2006) Management of sepsis. N Engl J Med 355: 1699–1713. - PubMed
-
- Damas P, Canivet JL, de Groote D, Vrindts Y, Albert A, et al. (1997) Sepsis and serum cytokine concentrations. Crit Care Med 25: 405–412. - PubMed
-
- Buras JA, Rice L, Orlow D, Pavlides S, Reenstra WR, et al. (2004) Inhibition of C5 or absence of C6 protects from sepsis mortality. Immunobiology 209: 629–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

