LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-γ production by repressing β-catenin activation in dendritic cells
- PMID: 23675302
- PMCID: PMC3649983
- DOI: 10.1371/journal.ppat.1003357
LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-γ production by repressing β-catenin activation in dendritic cells
Abstract
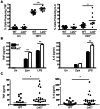
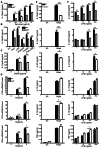
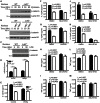
Fungal pathogens elicit cytokine responses downstream of immunoreceptor tyrosine-based activation motif (ITAM)-coupled or hemiITAM-containing receptors and TLRs. The Linker for Activation of B cells/Non-T cell Activating Linker (LAB/NTAL) encoded by Lat2, is a known regulator of ITAM-coupled receptors and TLR-associated cytokine responses. Here we demonstrate that LAB is involved in anti-fungal immunity. We show that Lat2-/- mice are more susceptible to C. albicans infection than wild type (WT) mice. Dendritic cells (DCs) express LAB and we show that it is basally phosphorylated by the growth factor M-CSF or following engagement of Dectin-2, but not Dectin-1. Our data revealed a unique mechanism whereby LAB controls basal and fungal/pathogen-associated molecular patterns (PAMP)-induced nuclear β-catenin levels. This in turn is important for controlling fungal/PAMP-induced cytokine production in DCs. C. albicans- and LPS-induced IL-12 and IL-23 production was blunted in Lat2-/- DCs. Accordingly, Lat2-/- DCs directed reduced Th1 polarization in vitro and Lat2-/- mice displayed reduced Natural Killer (NK) and T cell-mediated IFN-γ production in vivo/ex vivo. Thus our data define a novel link between LAB and β-catenin nuclear accumulation in DCs that facilitates IFN-γ responses during anti-fungal immunity. In addition, these findings are likely to be relevant to other infectious diseases that require IL-12 family cytokines and an IFN-γ response for pathogen clearance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




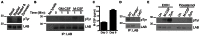

Similar articles
-
Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans.Immunity. 2013 Jun 27;38(6):1176-86. doi: 10.1016/j.immuni.2013.05.010. Epub 2013 Jun 13. Immunity. 2013. PMID: 23770228
-
The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells.J Biol Chem. 2010 Jan 29;285(5):2976-85. doi: 10.1074/jbc.M109.038398. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948717 Free PMC article.
-
Analysis of the linker for activation of T cells and the linker for activation of B cells in natural killer cells reveals a novel signaling cassette, dual usage in ITAM signaling, and influence on development of the Ly49 repertoire.Blood. 2008 Oct 1;112(7):2869-77. doi: 10.1182/blood-2007-11-121590. Epub 2008 Jul 21. Blood. 2008. PMID: 18645037 Free PMC article.
-
β-Catenin stabilization in NOD dendritic cells increases IL-12 production and subsequent induction of IFN-γ-producing T cells.J Leukoc Biol. 2019 Dec;106(6):1349-1358. doi: 10.1002/JLB.3A0919-244R. Epub 2019 Sep 30. J Leukoc Biol. 2019. PMID: 31568613
-
Ntal/Lab/Lat2.Int J Biochem Cell Biol. 2007;39(5):868-73. doi: 10.1016/j.biocel.2006.10.018. Epub 2006 Oct 28. Int J Biochem Cell Biol. 2007. PMID: 17118694 Free PMC article. Review.
Cited by
-
Leishmania donovani inhibits macrophage apoptosis and pro-inflammatory response through AKT-mediated regulation of β-catenin and FOXO-1.Cell Death Differ. 2016 Nov 1;23(11):1815-1826. doi: 10.1038/cdd.2016.101. Epub 2016 Sep 23. Cell Death Differ. 2016. PMID: 27662364 Free PMC article.
-
Emerging IL-12 family cytokines in the fight against fungal infections.Cytokine. 2018 Nov;111:398-407. doi: 10.1016/j.cyto.2018.05.019. Epub 2018 May 21. Cytokine. 2018. PMID: 29793796 Free PMC article. Review.
-
Dependence on Mincle and Dectin-2 Varies With Multiple Candida Species During Systemic Infection.Front Microbiol. 2021 Feb 25;12:633229. doi: 10.3389/fmicb.2021.633229. eCollection 2021. Front Microbiol. 2021. PMID: 33717025 Free PMC article.
-
β-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2823-8. doi: 10.1073/pnas.1414167112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25730849 Free PMC article.
-
CAWS administration increases the expression of interferon γ and complement factors that lead to severe vasculitis in DBA/2 mice.BMC Immunol. 2013 Sep 24;14:44. doi: 10.1186/1471-2172-14-44. BMC Immunol. 2013. PMID: 24063402 Free PMC article.
References
-
- Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36: 1–53. - PubMed
-
- Hamad M (2008) Antifungal immunotherapy and immunomodulation: a double-hitter approach to deal with invasive fungal infections. Scandinavian journal of immunology 67: 533–543. - PubMed
-
- Lehrnbecher T, Tramsen L, Koehl U, Schmidt S, Bochennek K, et al. (2011) Immunotherapy against invasive fungal diseases in stem cell transplant recipients. Immunological investigations 40: 839–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

