T-Cell tropism of simian varicella virus during primary infection
- PMID: 23675304
- PMCID: PMC3649965
- DOI: 10.1371/journal.ppat.1003368
T-Cell tropism of simian varicella virus during primary infection
Abstract
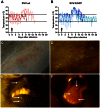
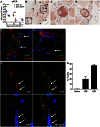
Varicella-zoster virus (VZV) causes varicella, establishes a life-long latent infection of ganglia and reactivates to cause herpes zoster. The cell types that transport VZV from the respiratory tract to skin and ganglia during primary infection are unknown. Clinical, pathological, virological and immunological features of simian varicella virus (SVV) infection of non-human primates parallel those of primary VZV infection in humans. To identify the host cell types involved in virus dissemination and pathology, we infected African green monkeys intratracheally with recombinant SVV expressing enhanced green fluorescent protein (SVV-EGFP) and with wild-type SVV (SVV-wt) as a control. The SVV-infected cell types and virus kinetics were determined by flow cytometry and immunohistochemistry, and virus culture and SVV-specific real-time PCR, respectively. All monkeys developed fever and skin rash. Except for pneumonitis, pathology produced by SVV-EGFP was less compared to SVV-wt. In lungs, SVV infected alveolar myeloid cells and T-cells. During viremia the virus preferentially infected memory T-cells, initially central memory T-cells and subsequently effector memory T-cells. In early non-vesicular stages of varicella, SVV was seen mainly in perivascular skin infiltrates composed of macrophages, dendritic cells, dendrocytes and memory T-cells, implicating hematogenous spread. In ganglia, SVV was found primarily in neurons and occasionally in memory T-cells adjacent to neurons. In conclusion, the data suggest the role of memory T-cells in disseminating SVV to its target organs during primary infection of its natural and immunocompetent host.
Conflict of interest statement
Co-author ADMEO wishes to declare, for the avoidance of any misunderstanding on competing interests, that he co-founded and is chief scientific officer of Viroclinics Biosciences, a company set up in collaboration with Erasmus MC. However, for clarification, no materials or support were received from the company, and no agreements were in place concerning the execution or publication of this work. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials. The authors have no additional financial interests.
Figures


Similar articles
-
Simian varicella virus infection of Chinese rhesus macaques produces ganglionic infection in the absence of rash.J Neurovirol. 2012 Apr;18(2):91-9. doi: 10.1007/s13365-012-0083-4. Epub 2012 Mar 8. J Neurovirol. 2012. PMID: 22399159 Free PMC article.
-
Simian Varicella Virus Is Present in Macrophages, Dendritic Cells, and T Cells in Lymph Nodes of Rhesus Macaques after Experimental Reactivation.J Virol. 2015 Oct;89(19):9817-24. doi: 10.1128/JVI.01324-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178993 Free PMC article.
-
Simian varicella: a model for human varicella-zoster virus infections.Rev Med Virol. 2004 Nov-Dec;14(6):363-81. doi: 10.1002/rmv.437. Rev Med Virol. 2004. PMID: 15386593 Review.
-
Abortive intrabronchial infection of rhesus macaques with varicella-zoster virus provides partial protection against simian varicella virus challenge.J Virol. 2015 Feb;89(3):1781-93. doi: 10.1128/JVI.03124-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410871 Free PMC article.
-
Varicella virus-mononuclear cell interaction.Adv Virus Res. 2003;62:1-17. doi: 10.1016/s0065-3527(03)62001-4. Adv Virus Res. 2003. PMID: 14719363 Review.
Cited by
-
Direct transfer of viral and cellular proteins from varicella-zoster virus-infected non-neuronal cells to human axons.PLoS One. 2015 May 14;10(5):e0126081. doi: 10.1371/journal.pone.0126081. eCollection 2015. PLoS One. 2015. PMID: 25973990 Free PMC article.
-
Immunobiology of Varicella-Zoster Virus Infection.J Infect Dis. 2018 Sep 22;218(suppl_2):S68-S74. doi: 10.1093/infdis/jiy403. J Infect Dis. 2018. PMID: 30247598 Free PMC article. Review.
-
Manipulation of the Innate Immune Response by Varicella Zoster Virus.Front Immunol. 2020 Jan 24;11:1. doi: 10.3389/fimmu.2020.00001. eCollection 2020. Front Immunol. 2020. PMID: 32038653 Free PMC article. Review.
-
Varicella Zoster Virus in the Nervous System.F1000Res. 2015 Nov 26;4:F1000 Faculty Rev-1356. doi: 10.12688/f1000research.7153.1. eCollection 2015. F1000Res. 2015. PMID: 26918131 Free PMC article. Review.
-
Simian varicella virus inhibits the interferon gamma signalling pathway.J Gen Virol. 2017 Oct;98(10):2582-2588. doi: 10.1099/jgv.0.000925. J Gen Virol. 2017. PMID: 28901902 Free PMC article.
References
-
- Cohen JI, Straus SE, Arvin AM (2007) Varicella-zoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edition. Philadelphia, PA: Lippincott-Williams and Wilkins. pp. 2773–2818.
-
- Grose C (1981) Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68: 735–737. - PubMed
-
- Heininger U, Seward JF (2006) Varicella. Lancet 368: 1365–1376. - PubMed
-
- Asano Y, Itakura N, Hiroishi Y, Hirose S, Ozaki T, et al. (1985) Viral replication and immunologic responses in children naturally infected with varicella-zoster virus and in varicella vaccine recipients. J Infect Dis 152: 863–868. - PubMed
-
- Koropchak CM, Graham G, Palmer J, Winsberg M, Ting SF, et al. (1991) Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis 163: 1016–1022. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

