Frequency response areas in the inferior colliculus: nonlinearity and binaural interaction
- PMID: 23675323
- PMCID: PMC3650518
- DOI: 10.3389/fncir.2013.00090
Frequency response areas in the inferior colliculus: nonlinearity and binaural interaction
Abstract
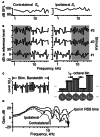
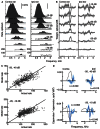
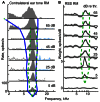
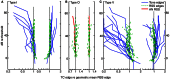
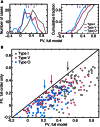
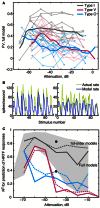
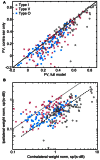
The tuning, binaural properties, and encoding characteristics of neurons in the central nucleus of the inferior colliculus (CNIC) were investigated to shed light on nonlinearities in the responses of these neurons. Results were analyzed for three types of neurons (I, O, and V) in the CNIC of decerebrate cats. Rate responses to binaural stimuli were characterized using a 1st- plus 2nd-order spectral integration model. Parameters of the model were derived using broadband stimuli with random spectral shapes (RSS). This method revealed four characteristics of CNIC neurons: (1) Tuning curves derived from broadband stimuli have fixed (i. e., level tolerant) bandwidths across a 50-60 dB range of sound levels; (2) 1st-order contralateral weights (particularly for type I and O neurons) were usually larger in magnitude than corresponding ipsilateral weights; (3) contralateral weights were more important than ipsilateral weights when using the model to predict responses to untrained noise stimuli; and (4) 2nd-order weight functions demonstrate frequency selectivity different from that of 1st-order weight functions. Furthermore, while the inclusion of 2nd-order terms in the model usually improved response predictions related to untrained RSS stimuli, they had limited impact on predictions related to other forms of filtered broadband noise [e. g., virtual-space stimuli (VS)]. The accuracy of the predictions varied considerably by response type. Predictions were most accurate for I neurons, and less accurate for O and V neurons, except at the lowest stimulus levels. These differences in prediction performance support the idea that type I, O, and V neurons encode different aspects of the stimulus: while type I neurons are most capable of producing linear representations of spectral shape, type O and V neurons may encode spectral features or temporal stimulus properties in a manner not easily explained with the low-order model. Supported by NIH grant DC00115.
Keywords: binaural; dynamic range; inferior colliculus; level tolerant; model; nonlinearity; random spectral shape; tuning.
Figures



Similar articles
-
Linear processing of interaural level difference underlies spatial tuning in the nucleus of the brachium of the inferior colliculus.J Neurosci. 2013 Feb 27;33(9):3891-904. doi: 10.1523/JNEUROSCI.3437-12.2013. J Neurosci. 2013. PMID: 23447600 Free PMC article.
-
Responses of inferior colliculus neurons to time-varying interaural phase disparity: effects of shifting the locus of virtual motion.J Neurophysiol. 1993 Apr;69(4):1245-63. doi: 10.1152/jn.1993.69.4.1245. J Neurophysiol. 1993. PMID: 8492161
-
Effects of interaural time delays of noise stimuli on low-frequency cells in the cat's inferior colliculus. II. Responses to band-pass filtered noises.J Neurophysiol. 1987 Sep;58(3):543-61. doi: 10.1152/jn.1987.58.3.543. J Neurophysiol. 1987. PMID: 3655882
-
Binaural inhibition is important in shaping the free-field frequency selectivity of single neurons in the inferior colliculus.J Neurophysiol. 1996 Oct;76(4):2580-94. doi: 10.1152/jn.1996.76.4.2580. J Neurophysiol. 1996. PMID: 8899629
-
Complex sound analysis (frequency resolution, filtering and spectral integration) by single units of the inferior colliculus of the cat.Brain Res. 1988 Apr-Jun;472(2):139-63. doi: 10.1016/0165-0173(88)90018-5. Brain Res. 1988. PMID: 3289688 Review.
Cited by
-
Sodium salicylate improves detection of amplitude-modulated sound in mice.iScience. 2024 Apr 9;27(5):109691. doi: 10.1016/j.isci.2024.109691. eCollection 2024 May 17. iScience. 2024. PMID: 38736549 Free PMC article.
-
Dorsal Cochlear Nucleus of the Rat: Representation of Complex Sounds in Ears Damaged by Acoustic Trauma.J Assoc Res Otolaryngol. 2015 Aug;16(4):487-505. doi: 10.1007/s10162-015-0522-z. Epub 2015 May 13. J Assoc Res Otolaryngol. 2015. PMID: 25967754 Free PMC article.
-
Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus.J Assoc Res Otolaryngol. 2014 Dec;15(6):1007-22. doi: 10.1007/s10162-014-0488-2. Epub 2014 Sep 26. J Assoc Res Otolaryngol. 2014. PMID: 25255865 Free PMC article.
-
Linear coding of complex sound spectra by discharge rate in neurons of the medial nucleus of the trapezoid body (MNTB) and its inputs.Front Neural Circuits. 2014 Dec 16;8:144. doi: 10.3389/fncir.2014.00144. eCollection 2014. Front Neural Circuits. 2014. PMID: 25565971 Free PMC article.
-
Stimulus-frequency-dependent dominance of sound localization cues across the cochleotopic map of the inferior colliculus.J Neurophysiol. 2020 May 1;123(5):1791-1807. doi: 10.1152/jn.00713.2019. Epub 2020 Mar 18. J Neurophysiol. 2020. PMID: 32186439 Free PMC article.
References
-
- Aertsen A. M. H. J., Johannesma P. I. M. (1981). The spectro-temporal receptive field. A functional characteristic of auditory neurons. Biol. Cybern. 42, 133–143 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

