Distinguishing synchronous and time-varying synergies using point process interval statistics: motor primitives in frog and rat
- PMID: 23675341
- PMCID: PMC3648693
- DOI: 10.3389/fncom.2013.00052
Distinguishing synchronous and time-varying synergies using point process interval statistics: motor primitives in frog and rat
Abstract
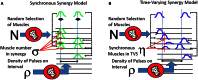
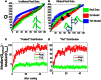
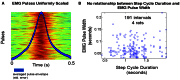
We present and apply a method that uses point process statistics to discriminate the forms of synergies in motor pattern data, prior to explicit synergy extraction. The method uses electromyogram (EMG) pulse peak timing or onset timing. Peak timing is preferable in complex patterns where pulse onsets may be overlapping. An interval statistic derived from the point processes of EMG peak timings distinguishes time-varying synergies from synchronous synergies (SS). Model data shows that the statistic is robust for most conditions. Its application to both frog hindlimb EMG and rat locomotion hindlimb EMG show data from these preparations is clearly most consistent with synchronous synergy models (p < 0.001). Additional direct tests of pulse and interval relations in frog data further bolster the support for synchronous synergy mechanisms in these data. Our method and analyses support separated control of rhythm and pattern of motor primitives, with the low level execution primitives comprising pulsed SS in both frog and rat, and both episodic and rhythmic behaviors.
Keywords: point process; primitives; synchronous synergy; synergy; time-varying synergy.
Figures






Similar articles
-
Motor primitives are determined in early development and are then robustly conserved into adulthood.Proc Natl Acad Sci U S A. 2019 Jun 11;116(24):12025-12034. doi: 10.1073/pnas.1821455116. Epub 2019 May 28. Proc Natl Acad Sci U S A. 2019. PMID: 31138689 Free PMC article.
-
Between-subject variability of muscle synergies during a complex motor skill.Front Comput Neurosci. 2012 Dec 28;6:99. doi: 10.3389/fncom.2012.00099. eCollection 2012. Front Comput Neurosci. 2012. PMID: 23293599 Free PMC article.
-
Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog.J Neurophysiol. 2001 Feb;85(2):605-19. doi: 10.1152/jn.2001.85.2.605. J Neurophysiol. 2001. PMID: 11160497
-
Motor primitives and synergies in the spinal cord and after injury--the current state of play.Ann N Y Acad Sci. 2013 Mar;1279:114-26. doi: 10.1111/nyas.12065. Ann N Y Acad Sci. 2013. PMID: 23531009 Free PMC article. Review.
-
Neuromuscular synergies in motor control in normal and poststroke individuals.Rev Neurosci. 2018 Aug 28;29(6):593-612. doi: 10.1515/revneuro-2017-0058. Rev Neurosci. 2018. PMID: 29397390 Review.
Cited by
-
A three-dimensional musculoskeletal model of the dog.Sci Rep. 2021 May 31;11(1):11335. doi: 10.1038/s41598-021-90058-0. Sci Rep. 2021. PMID: 34059703 Free PMC article.
-
Model selection for the extraction of movement primitives.Front Comput Neurosci. 2013 Dec 20;7:185. doi: 10.3389/fncom.2013.00185. eCollection 2013. Front Comput Neurosci. 2013. PMID: 24391580 Free PMC article.
-
Making the Coupled Gaussian Process Dynamical Model Modular and Scalable with Variational Approximations.Entropy (Basel). 2018 Sep 21;20(10):724. doi: 10.3390/e20100724. Entropy (Basel). 2018. PMID: 33265813 Free PMC article.
-
Muscle synergies are associated with intermuscular coherence and cortico-synergy coherence in an isometric upper limb task.Exp Brain Res. 2023 Dec;241(11-12):2627-2643. doi: 10.1007/s00221-023-06706-6. Epub 2023 Sep 22. Exp Brain Res. 2023. PMID: 37737925 Free PMC article.
-
Neuromechanical Model of Rat Hindlimb Walking with Two-Layer CPGs.Biomimetics (Basel). 2019 Mar 1;4(1):21. doi: 10.3390/biomimetics4010021. Biomimetics (Basel). 2019. PMID: 31105206 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

