Upregulation of miR-150* and miR-630 induces apoptosis in pancreatic cancer cells by targeting IGF-1R
- PMID: 23675407
- PMCID: PMC3651232
- DOI: 10.1371/journal.pone.0061015
Upregulation of miR-150* and miR-630 induces apoptosis in pancreatic cancer cells by targeting IGF-1R
Abstract
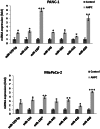
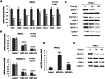
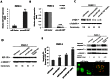
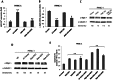
MicroRNAs have been implicated in many critical cellular processes including apoptosis. We have previously found that apoptosis in pancreatic cancer cells was induced by adamantyl retinoid-related (ARR) molecule 3-Cl-AHPC. Here we report that 3-Cl-AHPC-dependent apoptosis involves regulating a number of microRNAs including miR-150* and miR-630. 3-Cl-AHPC stimulated miR-150* expression and caused decreased expression of c-Myb and IGF-1R in the pancreatic cancer cells. 3-Cl-AHPC-mediated reduction of c-Myb resulted in diminished binding of c-Myb with IGF-1R and Bcl-2 promoters, thereby causing repression of their transcription and protein expression. Over-expression of miR-150* also resulted in diminished levels of c-Myb and Bcl-2 proteins. Furthermore, the addition of the miRNA inhibitor 2'-O-methylated miR-150 blocked 3-Cl-AHPC-mediated increase in miR-150* levels and abrogated loss of c-Myb protein. Knockdown of c-Myb in PANC-1 cells resulted in enhanced apoptosis both in the presence or absence of 3-Cl-AHPC confirming the anti-apoptotic property of c-Myb. Overexpression of miR-630 also induced apoptosis in the pancreatic cancer cells and inhibited target protein IGF-1R mRNA and protein expression. Together these results implicate key roles for miR-150* and miR-630 and their targeting of IGF-1R to promote apoptosis in pancreatic cancer cells.
Conflict of interest statement
Figures


Similar articles
-
Down regulation of miR-202 modulates Mxd1 and Sin3A repressor complexes to induce apoptosis of pancreatic cancer cells.Cancer Biol Ther. 2015;16(1):115-24. doi: 10.4161/15384047.2014.987070. Cancer Biol Ther. 2015. PMID: 25611699 Free PMC article.
-
microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb.Int J Oncol. 2012 Mar;40(3):747-56. doi: 10.3892/ijo.2011.1242. Epub 2011 Oct 24. Int J Oncol. 2012. PMID: 22025269
-
SHP and Sin3A expression are essential for adamantyl-substituted retinoid-related molecule-mediated nuclear factor-kappaB activation, c-Fos/c-Jun expression, and cellular apoptosis.Mol Cancer Ther. 2009 Jun;8(6):1625-35. doi: 10.1158/1535-7163.MCT-08-0964. Epub 2009 Jun 9. Mol Cancer Ther. 2009. PMID: 19509248
-
MicroRNA-302b-3p Suppresses Cell Proliferation Through AKT Pathway by Targeting IGF-1R in Human Gastric Cancer.Cell Physiol Biochem. 2017;42(4):1701-1711. doi: 10.1159/000479419. Epub 2017 Jul 25. Cell Physiol Biochem. 2017. PMID: 28743112
-
Potential role of microRNAs in pancreatic cancer manifestation: a review.J Egypt Natl Canc Inst. 2022 Jun 20;34(1):26. doi: 10.1186/s43046-022-00127-2. J Egypt Natl Canc Inst. 2022. PMID: 35718815 Review.
Cited by
-
Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2.Acta Pharm Sin B. 2019 Sep;9(5):1008-1020. doi: 10.1016/j.apsb.2019.01.002. Epub 2019 Jan 8. Acta Pharm Sin B. 2019. PMID: 31649850 Free PMC article.
-
Deep Sequencing the microRNA profile in rhabdomyosarcoma reveals down-regulation of miR-378 family members.BMC Cancer. 2014 Nov 25;14:880. doi: 10.1186/1471-2407-14-880. BMC Cancer. 2014. PMID: 25427715 Free PMC article.
-
miR-630 targets LMO3 to regulate cell growth and metastasis in lung cancer.Am J Transl Res. 2015 Jul 15;7(7):1271-9. eCollection 2015. Am J Transl Res. 2015. PMID: 26328011 Free PMC article.
-
MicroRNA-630 suppresses tumor metastasis through the TGF-β- miR-630-Slug signaling pathway and correlates inversely with poor prognosis in hepatocellular carcinoma.Oncotarget. 2016 Apr 19;7(16):22674-86. doi: 10.18632/oncotarget.8047. Oncotarget. 2016. PMID: 26993767 Free PMC article.
-
miR-630 functions as a tumor oncogene in renal cell carcinoma.Arch Med Sci. 2016 Jun 1;12(3):473-8. doi: 10.5114/aoms.2016.59918. Epub 2016 May 18. Arch Med Sci. 2016. PMID: 27279836 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

