Diagnosis and interim treatment outcomes from the first cohort of multidrug-resistant tuberculosis patients in Tanzania
- PMID: 23675411
- PMCID: PMC3652861
- DOI: 10.1371/journal.pone.0062034
Diagnosis and interim treatment outcomes from the first cohort of multidrug-resistant tuberculosis patients in Tanzania
Abstract
Setting: Kibong'oto National Tuberculosis Hospital (KNTH), Kilimanjaro, Tanzania.
Objective: Characterize the diagnostic process and interim treatment outcomes from patients treated for multidrug-resistant tuberculosis (MDR-TB) in Tanzania.
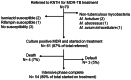
Design: A retrospective cohort study was performed among all patients treated at KNTH for pulmonary MDR-TB between November 2009 and September 2011.
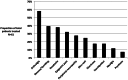
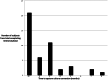
Results: Sixty-one culture-positive MDR-TB patients initiated therapy, 60 (98%) with a prior history of TB treatment. Forty-one (67%) were male and 9 (14%) were HIV infected with a mean CD4 count of 424 (±106) cells/µl. The median time from specimen collection to MDR-TB diagnosis and from diagnosis to initiation of MDR-TB treatment was 138 days (IQR 101-159) and 131 days (IQR 32-233), respectively. Following treatment initiation four (7%) patients died (all HIV negative), 3 (5%) defaulted, and the remaining 54 (89%) completed the intensive phase. Most adverse drug reactions were mild to moderate and did not require discontinuation of treatment. Median time to culture conversion was 2 months (IQR 1-3) and did not vary by HIV status. In 28 isolates available for additional second-line drug susceptibility testing, fluoroquinolone, aminoglycoside and para-aminosalicylic acid resistance was rare yet ethionamide resistance was present in 9 (32%).
Conclusion: The majority of MDR-TB patients from this cohort had survived a prolonged referral process, had multiple episodes of prior TB treatment, but did not have advanced AIDS and converted to culture negative early while completing an intensive inpatient regimen without serious adverse event. Further study is required to determine the clinical impact of second-line drug susceptibility testing and the feasibility of alternatives to prolonged hospitalization.
Conflict of interest statement
Figures
Similar articles
-
Application of quantitative second-line drug susceptibility testing at a multidrug-resistant tuberculosis hospital in Tanzania.BMC Infect Dis. 2013 Sep 14;13:432. doi: 10.1186/1471-2334-13-432. BMC Infect Dis. 2013. PMID: 24034230 Free PMC article.
-
Potentially High Number of Ineffective Drugs with the Standard Shorter Course Regimen for Multidrug-Resistant Tuberculosis Treatment in Haiti.Am J Trop Med Hyg. 2019 Feb;100(2):392-398. doi: 10.4269/ajtmh.18-0493. Am J Trop Med Hyg. 2019. PMID: 30594266 Free PMC article.
-
Treatment outcomes of rifampin-sparing treatment in patients with pulmonary tuberculosis with rifampin-mono-resistance or rifampin adverse events: A retrospective cohort analysis.Respir Med. 2017 Oct;131:43-48. doi: 10.1016/j.rmed.2017.08.002. Epub 2017 Aug 4. Respir Med. 2017. PMID: 28947041
-
Treatment and outcomes in children with multidrug-resistant tuberculosis: A systematic review and individual patient data meta-analysis.PLoS Med. 2018 Jul 11;15(7):e1002591. doi: 10.1371/journal.pmed.1002591. eCollection 2018 Jul. PLoS Med. 2018. PMID: 29995958 Free PMC article.
-
Is hypothyroidism rare in multidrug resistance tuberculosis patients on treatment? A systematic review and meta-analysis.PLoS One. 2019 Jun 18;14(6):e0218487. doi: 10.1371/journal.pone.0218487. eCollection 2019. PLoS One. 2019. PMID: 31211809 Free PMC article.
Cited by
-
Application of quantitative second-line drug susceptibility testing at a multidrug-resistant tuberculosis hospital in Tanzania.BMC Infect Dis. 2013 Sep 14;13:432. doi: 10.1186/1471-2334-13-432. BMC Infect Dis. 2013. PMID: 24034230 Free PMC article.
-
Plasma drug activity in patients on treatment for multidrug-resistant tuberculosis.Antimicrob Agents Chemother. 2014;58(2):782-8. doi: 10.1128/AAC.01549-13. Epub 2013 Nov 18. Antimicrob Agents Chemother. 2014. PMID: 24247125 Free PMC article.
-
Underestimated pyrazinamide resistance may compromise outcomes of pyrazinamide containing regimens for treatment of drug susceptible and multi-drug-resistant tuberculosis in Tanzania.BMC Infect Dis. 2019 Feb 7;19(1):129. doi: 10.1186/s12879-019-3757-1. BMC Infect Dis. 2019. PMID: 30732572 Free PMC article.
-
Gridlock from diagnosis to treatment of multidrug resistant tuberculosis (MDR-TB) in Tanzania: patients' perspectives from a focus group discussion.BMC Public Health. 2020 Nov 7;20(1):1667. doi: 10.1186/s12889-020-09774-3. BMC Public Health. 2020. PMID: 33160327 Free PMC article.
-
High rates of culture conversion and low loss to follow-up in MDR-TB patients managed at Regional Referral Hospitals in Uganda.BMC Infect Dis. 2021 Oct 12;21(1):1060. doi: 10.1186/s12879-021-06743-y. BMC Infect Dis. 2021. PMID: 34641816 Free PMC article.
References
-
- Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, et al. (2010) Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. The Lancet 375: 1830–1843. - PubMed
-
- World Health Organization (2010) Global tuberculosis control. Geneva, World Health Organization.
-
- World Health Organization (2011) Guidelines for the programmatic management of drug-resistant tuberculosis, Update. Geneva, World Health Organization. - PubMed
-
- Chonde TM, Basra D, Mfinanga SG, Range N, Lwilla F, et al. (2010) National anti-tuberculosis drug resistance study in Tanzania. Int J Tuberc Lung Dis 14: 967–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

