Basolateral BMP signaling in polarized epithelial cells
- PMID: 23675417
- PMCID: PMC3652834
- DOI: 10.1371/journal.pone.0062659
Basolateral BMP signaling in polarized epithelial cells
Abstract
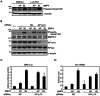
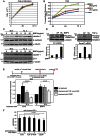
Bone morphogenetic proteins (BMPs) regulate various biological processes, mostly mediated by cells of mesenchymal origin. However, the roles of BMPs in epithelial cells are poorly understood. Here, we demonstrate that, in polarized epithelial cells, BMP signals are transmitted from BMP receptor complexes exclusively localized at the basolateral surface of the cell membrane. In addition, basolateral stimulation with BMP increased expression of components of tight junctions and enhanced the transepithelial resistance (TER), counteracting reduction of TER by treatment with TGF-β or an anti-tumor drug. We conclude that BMPs maintain epithelial polarity via intracellular signaling from basolaterally localized BMP receptors.
Conflict of interest statement
Figures


Similar articles
-
Biphasic effects of transforming growth factor β on bone morphogenetic protein-induced osteoblast differentiation.J Bone Miner Res. 2011 Jun;26(6):1178-87. doi: 10.1002/jbmr.313. J Bone Miner Res. 2011. PMID: 21611961
-
Burst BMP triggered receptor kinase activity drives Smad1 mediated long-term target gene oscillation in C2C12 cells.PLoS One. 2013;8(4):e59442. doi: 10.1371/journal.pone.0059442. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23560048 Free PMC article.
-
AMOT130 drives BMP-SMAD signaling at the apical membrane in polarized cells.Mol Biol Cell. 2020 Jan 15;31(2):118-130. doi: 10.1091/mbc.E19-03-0179. Epub 2019 Dec 4. Mol Biol Cell. 2020. PMID: 31800378 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
[Advances in osteogenic mechanism and osteogenic effects of bone morphogenetic protein 6].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Sep;27(9):1144-7. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24279032 Review. Chinese.
Cited by
-
Deletion/loss of bone morphogenetic protein 7 changes tooth morphology and function in Mus musculus: implications for dental evolution in mammals.R Soc Open Sci. 2018 Jan 3;5(1):170761. doi: 10.1098/rsos.170761. eCollection 2018 Jan. R Soc Open Sci. 2018. PMID: 29410800 Free PMC article.
-
TGF-β Family Signaling in Ductal Differentiation and Branching Morphogenesis.Cold Spring Harb Perspect Biol. 2018 Mar 1;10(3):a031997. doi: 10.1101/cshperspect.a031997. Cold Spring Harb Perspect Biol. 2018. PMID: 28289061 Free PMC article. Review.
-
Apical Secretion of FSTL1 in the Respiratory Epithelium for Normal Lung Development.PLoS One. 2016 Jun 29;11(6):e0158385. doi: 10.1371/journal.pone.0158385. eCollection 2016. PLoS One. 2016. PMID: 27355685 Free PMC article.
-
A transient wave of BMP signaling in the retina is necessary for Müller glial differentiation.Development. 2015 Feb 1;142(3):533-43. doi: 10.1242/dev.118745. Development. 2015. PMID: 25605781 Free PMC article.
-
A Survey of Strategies to Modulate the Bone Morphogenetic Protein Signaling Pathway: Current and Future Perspectives.Stem Cells Int. 2016;2016:7290686. doi: 10.1155/2016/7290686. Epub 2016 Jun 28. Stem Cells Int. 2016. PMID: 27433166 Free PMC article. Review.
References
-
- Keita AV, Soderholm JD (2012) Barrier dysfunction and bacterial uptake in the follicle-associated epithelium of ileal Crohn’s disease. Ann. N. Y. Acad. Sci. 1258: 125–134. - PubMed
-
- Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2: 285–293. - PubMed
-
- Rodriguez-Boulan E, Kreitzer G, Musch A (2005) Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6: 233–247. - PubMed
-
- Folsch H (2005) The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 15: 222–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

