Targeting caspase-3 as dual therapeutic benefits by RNAi facilitating brain-targeted nanoparticles in a rat model of Parkinson's disease
- PMID: 23675438
- PMCID: PMC3652845
- DOI: 10.1371/journal.pone.0062905
Targeting caspase-3 as dual therapeutic benefits by RNAi facilitating brain-targeted nanoparticles in a rat model of Parkinson's disease
Erratum in
- PLoS One. 2013;8(9). doi:10.1371/annotation/5f08fe1e-8868-421c-92ea-1a4aa987d11f. Lv, Jing [corrected to Lu, Jing]
Abstract
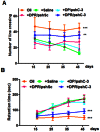
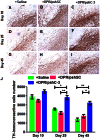
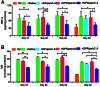
The activation of caspase-3 is an important hallmark in Parkinson's disease. It could induce neuron death by apoptosis and microglia activation by inflammation. As a result, inhibition the activation of caspase-3 would exert synergistic dual effect in brain in order to prevent the progress of Parkinson's disease. Silencing caspase-3 genes by RNA interference could inhibit the activation of caspase-3. We developed a brain-targeted gene delivery system based on non-viral gene vector, dendrigraft poly-L-lysines. A rabies virus glycoprotein peptide with 29 amino-acid linked to dendrigraft poly-L-lysines could render gene vectors the ability to get across the blood brain barrier by specific receptor mediated transcytosis. The resultant brain-targeted vector was complexed with caspase-3 short hairpin RNA coding plasmid DNA, yielding nanoparticles. In vivo imaging analysis indicated the targeted nanoparticles could accumulate in brain more efficiently than non-targeted ones. A multiple dosing regimen by weekly intravenous administration of the nanoparticles could reduce activated casapse-3 levels, significantly improve locomotor activity and rescue dopaminergic neuronal loss and in Parkinson's disease rats' brain. These results indicated the rabies virus glycoprotein peptide modified brain-targeted nanoparticles were promising gene delivery system for RNA interference to achieve anti-apoptotic and anti-inflammation synergistic therapeutic effects by down-regulation the expression and activation of caspase-3.
Conflict of interest statement
Figures




Similar articles
-
Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer's disease.Biomaterials. 2015 Jan;38:61-71. doi: 10.1016/j.biomaterials.2014.10.068. Epub 2014 Nov 13. Biomaterials. 2015. PMID: 25457984
-
T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting.Int J Pharm. 2013 Sep 15;454(1):11-20. doi: 10.1016/j.ijpharm.2013.07.019. Epub 2013 Jul 15. Int J Pharm. 2013. PMID: 23867728
-
Brain-targeting delivery for RNAi neuroprotection against cerebral ischemia reperfusion injury.Biomaterials. 2013 Nov;34(35):8949-59. doi: 10.1016/j.biomaterials.2013.07.060. Epub 2013 Aug 19. Biomaterials. 2013. PMID: 23968852
-
Ultrasound targeted CNS gene delivery for Parkinson's disease treatment.J Control Release. 2017 Sep 10;261:246-262. doi: 10.1016/j.jconrel.2017.07.004. Epub 2017 Jul 8. J Control Release. 2017. PMID: 28690161 Review.
-
Nanotechnology As Potential Tool for siRNA Delivery in Parkinson's Disease.Curr Drug Targets. 2017 Nov 30;18(16):1866-1879. doi: 10.2174/1389450118666170321130003. Curr Drug Targets. 2017. PMID: 28325145 Review.
Cited by
-
Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier.Annu Rev Pharmacol Toxicol. 2015;55:613-31. doi: 10.1146/annurev-pharmtox-010814-124852. Epub 2014 Oct 8. Annu Rev Pharmacol Toxicol. 2015. PMID: 25340933 Free PMC article. Review.
-
Drug Delivery Systems for Imaging and Therapy of Parkinson's Disease.Curr Neuropharmacol. 2016;14(4):376-91. doi: 10.2174/1570159x14666151230124904. Curr Neuropharmacol. 2016. PMID: 26714584 Free PMC article. Review.
-
Anti-inflammatory, anti-apoptotic, and neuroprotective potentials of anethole in Parkinson's disease-like motor and non-motor symptoms induced by rotenone in rats.Metab Brain Dis. 2023 Aug;38(6):2159-2174. doi: 10.1007/s11011-023-01230-6. Epub 2023 May 19. Metab Brain Dis. 2023. PMID: 37204660
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
-
Gene therapy targeting mitochondrial pathway in Parkinson's disease.J Neural Transm (Vienna). 2017 Feb;124(2):193-207. doi: 10.1007/s00702-016-1616-4. Epub 2016 Sep 16. J Neural Transm (Vienna). 2017. PMID: 27638713 Review.
References
-
- Fahn S (2006) Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl 71: 1–15. - PubMed
-
- Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, et al. (2002) Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. J Neurosci 23: 10302–10312. - PMC - PubMed
-
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH (2010) Neurotrophic factor therapy for Parkinson’s disease. Prog Brain Res 237–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

