HIF-1α transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits
- PMID: 23675495
- PMCID: PMC3652809
- DOI: 10.1371/journal.pone.0063628
HIF-1α transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits
Abstract
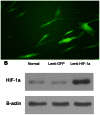
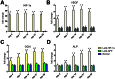
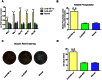
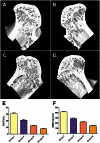
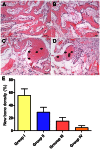
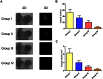
Although corticosteroid-induced osteonecrosis of the femoral head (ONFH) is common, the treatment for it remains limited and largely ineffective. We examined whether implantation of hypoxia inducible factor-1α (HIF-1α) transgenic bone marrow cells (BMCs) can promote the repair of the necrotic area of corticosteroid-induced ONFH. In this study, we confirmed that HIF-1α gene transfection could enhance mRNA expression of osteogenic genes in BMCs in vitro. Alkaline phosphatase activity assay and alizarin red-S staining indicated HIF-1α transgenic BMCs had enhanced osteogenic differentiation capacity in vitro. Furthermore, enzyme linked immunosorbent assay (ELISA) for VEGF revealed HIF-1α transgenic BMCs secreted more VEGF as compared to normal BMCs. An experimental rabbit model of early-stage corticosteroid-induced ONFH was established and used for an evaluation of cytotherapy. Transplantation of HIF-1α transgenic BMCs dramatically improved the bone regeneration of the necrotic area of the femoral head. The number and volume of blood vessel were significantly increased in the necrotic area of the femoral head compared to the control groups. These results support HIF-1α transgenic BMCs have enhanced osteogenic and angiogenic activity in vitro and in vivo. Transplantation of HIF-1α transgenic BMCs can potentially promote the repair of the necrotic area of corticosteroid-induced ONFH.
Conflict of interest statement
Figures


Similar articles
-
Hypoxia Enhanced Bone Regeneration Through the HIF-1α/β-Catenin Pathway in Femoral Head Osteonecrosis.Am J Med Sci. 2021 Jul;362(1):78-91. doi: 10.1016/j.amjms.2021.03.005. Epub 2021 Mar 13. Am J Med Sci. 2021. PMID: 33727018
-
Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit.Cell Biol Int. 2017 Dec;41(12):1379-1390. doi: 10.1002/cbin.10869. Epub 2017 Sep 25. Cell Biol Int. 2017. PMID: 28877384
-
Glucocorticoids induce femoral head necrosis in rats through the HIF-1α/VEGF signaling pathway.Sci Rep. 2025 Aug 9;15(1):29205. doi: 10.1038/s41598-025-15018-4. Sci Rep. 2025. PMID: 40783444 Free PMC article.
-
Application of biomaterials in treating early osteonecrosis of the femoral head: Research progress and future perspectives.Acta Biomater. 2023 Jul 1;164:15-73. doi: 10.1016/j.actbio.2023.04.005. Epub 2023 Apr 18. Acta Biomater. 2023. PMID: 37080444 Review.
-
Preclinical models for studying corticosteroid-induced osteonecrosis of the femoral head.J Biomed Mater Res B Appl Biomater. 2024 Jan;112(1):e35360. doi: 10.1002/jbm.b.35360. J Biomed Mater Res B Appl Biomater. 2024. PMID: 38247252 Review.
Cited by
-
Translational horizons in stem cell therapy for osteonecrosis of the femoral head: a journey from basic research to clinical practice through bibliometric insights.J Transl Med. 2024 Oct 30;22(1):982. doi: 10.1186/s12967-024-05784-6. J Transl Med. 2024. PMID: 39478610 Free PMC article.
-
Dimethyloxaloylglycine increases bone repair capacity of adipose-derived stem cells in the treatment of osteonecrosis of the femoral head.Exp Ther Med. 2016 Nov;12(5):2843-2850. doi: 10.3892/etm.2016.3698. Epub 2016 Sep 13. Exp Ther Med. 2016. PMID: 27882083 Free PMC article.
-
MiRNA inhibition in tissue engineering and regenerative medicine.Adv Drug Deliv Rev. 2015 Jul 1;88:123-37. doi: 10.1016/j.addr.2014.12.006. Epub 2014 Dec 29. Adv Drug Deliv Rev. 2015. PMID: 25553957 Free PMC article. Review.
-
Chrysophanic acid shifts the differentiation tendency of BMSCs to prevent alcohol-induced osteonecrosis of the femoral head.Cell Prolif. 2020 Aug;53(8):e12871. doi: 10.1111/cpr.12871. Epub 2020 Jun 29. Cell Prolif. 2020. PMID: 32597546 Free PMC article.
-
A Molecular Troika of Angiogenesis, Coagulopathy and Endothelial Dysfunction in the Pathology of Avascular Necrosis of Femoral Head: A Comprehensive Review.Cells. 2023 Sep 14;12(18):2278. doi: 10.3390/cells12182278. Cells. 2023. PMID: 37759498 Free PMC article. Review.
References
-
- Atsumi T, Kuroki Y (1992) Role of impairment of blood supply of the femoral head in the pathogenesis of idiopathic osteonecrosis. Clin Orthop Relat Res 277: 22–30. - PubMed
-
- Jones JP Jr (1993) Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res 292: 294–308. - PubMed
-
- Chiu KH, Shen WY, Ko CK, Chan KM (1997) Osteonecrosis of the femoral head treated with cementless total hip arthroplasty: a comparison with other diagnoses. J Arthroplasty 12: 683–688. - PubMed
-
- Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaqhan JJ, et al. (2003) Osteonecrosis of the hip: management in the 21st century. Instr Course Lect 52: 337–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

