Corticosterone facilitates fluoxetine-induced neuronal plasticity in the hippocampus
- PMID: 23675498
- PMCID: PMC3651130
- DOI: 10.1371/journal.pone.0063662
Corticosterone facilitates fluoxetine-induced neuronal plasticity in the hippocampus
Abstract
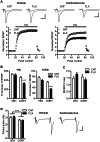
The hippocampal dentate gyrus has been implicated in a neuronal basis of antidepressant action. We have recently shown a distinct form of neuronal plasticity induced by the serotonergic antidepressant fluoxetine, that is, a reversal of maturation of the dentate granule cells in adult mice. This "dematuration" is induced in a large population of dentate neurons and maintained for at least one month after withdrawal of fluoxetine, suggesting long-lasting strong influence of dematuration on brain functioning. However, reliable induction of dematuration required doses of fluoxetine higher than suggested optimal doses for mice (10 to 18 mg/kg/day), which casts doubt on the clinical relevance of this effect. Since our previous studies were performed in naive mice, in the present study, we reexamined effects of fluoxetine using mice treated with chronic corticosterone that model neuroendocrine pathophysiology associated with depression. In corticosterone-treated mice, fluoxetine at 10 mg/kg/day downregulated expression of mature granule cell markers and attenuated strong frequency facilitation at the synapse formed by the granule cell axon mossy fiber, suggesting the induction of granule cell dematuration. In addition, fluoxetine caused marked enhancement of dopaminergic modulation at the mossy fiber synapse. In vehicle-treated mice, however, fluoxetine at this dose had no significant effects. The plasma level of fluoxetine was comparable to that in patients taking chronic fluoxetine, and corticosterone did not affect it. These results indicate that corticosterone facilitates fluoxetine-induced plastic changes in the dentate granule cells. Our finding may provide insight into neuronal mechanisms underlying enhanced responsiveness to antidepressant medication in certain pathological conditions.
Conflict of interest statement
Figures


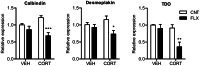
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

