A cluster randomized study of the safety of integrated treatment of trachoma and lymphatic filariasis in children and adults in Sikasso, Mali
- PMID: 23675549
- PMCID: PMC3649960
- DOI: 10.1371/journal.pntd.0002221
A cluster randomized study of the safety of integrated treatment of trachoma and lymphatic filariasis in children and adults in Sikasso, Mali
Abstract
Background: Neglected tropical diseases are co-endemic in many areas of the world, including sub Saharan Africa. Currently lymphatic filariasis (albendazole/ivermectin) and trachoma (azithromycin) are treated separately. Consequently, financial and logistical benefit can be gained from integration of preventive chemotherapy programs in such areas.
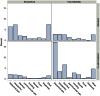
Methodology/findings: 4 villages in two co-endemic districts (Kolondièba and Bougouni) of Sikasso, Mali, were randomly assigned to coadministered treatment (ivermectin/albendazole/azithromycin) or standard therapy (ivermectin/albendazole with azithromycin 1 week later). These villages had previously undergone 4 annual MDA campaigns with ivermectin/albendazole and 2 with azithromycin. One village was randomly assigned to each treatment arm in each district. There were 7515 eligible individuals in the 4 villages, 3011(40.1%) of whom participated in the study. No serious adverse events occurred, and the majority of adverse events were mild in intensity (mainly headache, abdominal pain, diarrhoea and "other signs/symptoms"). The median time to the onset of the first event, of any type, was later (8 days) in the two standard treatment villages than in the co-administration villages. Overall the number of subjects reporting any event was similar in the co-administration group compared to the standard treatment group [18.7% (281/1501) vs. 15.8% (239/1510)]. However, the event frequency was higher in the coadministration group (30.4%) than in the standard treatment group (11.0%) in Kolondièba, while the opposite was observed in Bougouni (7.1% and 20.9% respectively). Additionally, the overall frequency of adverse events in the co-administration group (18.7%) was comparable to or lower than published frequencies for ivermectin+albendazole alone.
Conclusions: These data suggest that co-administration of ivermectin+albendazole and azithromycin is safe; however the small number of villages studied and the large differences between them resulted in an inability to calculate a meaningful overall estimate of the difference in adverse event rates between the regimens. Further work is therefore needed before co-administration can be definitively recommended.
Trial registration: ClinicalTrials.gov; NCT01586169.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96: 614–618. - PubMed
-
- Knirsch C (2007) Trachoma: ancient scourge, disease elimination, and future research. Curr Infect Dis Rep 9: 21–28. - PubMed
-
- Fry AM, Jha HC, Lietman TM, Chaudhary JS, Bhatta RC, et al. (2002) Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 35: 395–402. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

