Aging- and activation-induced platelet microparticles suppress apoptosis in monocytic cells and differentially signal to proinflammatory mediator release
- PMID: 23675563
- PMCID: PMC3649808
Aging- and activation-induced platelet microparticles suppress apoptosis in monocytic cells and differentially signal to proinflammatory mediator release
Abstract
Background: Platelet microparticles (PM) are the most abundant cell-derived microparticles in the blood, and accumulate in thrombo-inflammatory diseases. Platelets produce PM upon aging via an apoptosis-like process and by activation with strong agonists. We previously showed that long-term treatment of monocytic cells with apoptosis-induced PM (PMap) promotes their differentiation into resident macrophages. Here we investigated shorter term effects of various types of PM on monocyte signalling and function.
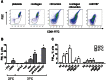
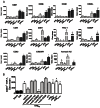
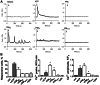
Methods and results: Flow cytometry and scanning electron microscopy revealed that PM formed upon platelet aging (PMap) or ultra-sonication (PMsonic) expressed activated αIIbβ3 integrins and tended to assemble into aggregates. In contrast, PM formed upon platelet activation with thrombin (PMthr) or Ca(2+) ionophore (PMiono) had mostly non-activated αIIbβ3 and little aggregate formation, but had increased CD63 expression. PM from activated and sonicated platelets expressed phosphatidylserine at their surface, while only the latter were enriched in the receptors CD40L and CX3CR1. All PM types expressed P-selectin, interacted with monocytic cells via this receptor, and were internalised into these cells. The various PM types promoted actin cytoskeletal rearrangements and hydrogen peroxide production by monocytic cells. Markedly, both aging- and activation-induced PM types stimulated the phosphoinositide 3-kinase/Akt pathway, suppressing apoptosis induced by several agonists, in a P-selectin-dependent manner. On the other hand, the PM types differentially influenced monocyte signalling in eliciting Ca(2+) fluxes (particularly PMap) and in releasing secondary mediators (complement factor C5a with PMap, and pro-inflammatory tumour necrosis factor-α with PMthr).
Conclusions: In spite of their common anti-apoptotic potential via Akt activation, aging- and activation-induced PM cause different Ca(2+) signalling events and mediator release in monocytic cells. By implication, aging and activated platelets may modulate monocyte function in different way by the shedding of different PM types.
Keywords: Aging; apoptosis; microparticles; monocytes; platelet activation; tumour necrosis factor.
Figures





Similar articles
-
Microparticles from apoptotic platelets promote resident macrophage differentiation.Cell Death Dis. 2011 Sep 29;2(9):e211. doi: 10.1038/cddis.2011.94. Cell Death Dis. 2011. PMID: 21956548 Free PMC article.
-
Staphylococcal SSL5-induced platelet microparticles provoke proinflammatory responses via the CD40/TRAF6/NFκB signalling pathway in monocytes.Thromb Haemost. 2016 Mar;115(3):632-45. doi: 10.1160/TH15-04-0322. Epub 2015 Dec 3. Thromb Haemost. 2016. PMID: 26632487
-
Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles.J Thromb Haemost. 2022 May;20(5):1223-1235. doi: 10.1111/jth.15668. Epub 2022 Feb 21. J Thromb Haemost. 2022. PMID: 35146910 Free PMC article.
-
Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability.J Thromb Haemost. 2011 Nov;9(11):2251-61. doi: 10.1111/j.1538-7836.2011.04488.x. J Thromb Haemost. 2011. PMID: 21883880 Free PMC article.
-
FLow-induced PRotrusions (FLIPRs): a platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils.Circ Res. 2014 Feb 28;114(5):780-91. doi: 10.1161/CIRCRESAHA.114.302361. Epub 2014 Jan 9. Circ Res. 2014. PMID: 24406984
Cited by
-
Extracellular Vesicles: Versatile Nanomediators, Potential Biomarkers and Therapeutic Agents in Atherosclerosis and COVID-19-Related Thrombosis.Int J Mol Sci. 2021 May 31;22(11):5967. doi: 10.3390/ijms22115967. Int J Mol Sci. 2021. PMID: 34073119 Free PMC article. Review.
-
Microparticles as potential biomarkers of cardiovascular disease.Arq Bras Cardiol. 2015 Feb;104(2):169-74. doi: 10.5935/abc.20140210. Epub 2015 Jan 27. Arq Bras Cardiol. 2015. PMID: 25626759 Free PMC article. Review.
-
Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis.Front Pharmacol. 2016 Aug 31;7:293. doi: 10.3389/fphar.2016.00293. eCollection 2016. Front Pharmacol. 2016. PMID: 27630570 Free PMC article. Review.
-
Differential regulation of the apoptotic machinery during megakaryocyte differentiation and platelet production by inhibitor of apoptosis protein Livin.Cell Death Dis. 2013 Nov 28;4(11):e937. doi: 10.1038/cddis.2013.454. Cell Death Dis. 2013. PMID: 24287698 Free PMC article.
-
Platelet Microparticles Protect Acute Myelogenous Leukemia Cells against Daunorubicin-Induced Apoptosis.Cancers (Basel). 2021 Apr 14;13(8):1870. doi: 10.3390/cancers13081870. Cancers (Basel). 2021. PMID: 33919720 Free PMC article.
References
-
- Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. - PubMed
-
- Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. - PubMed
-
- Koga H, Sugiyama S, Kugiyama K, Fukushima H, Watanabe K, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27:817–823. - PubMed
-
- Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;1:8–23. - PubMed
-
- Vasina E, Heemskerk JW, Weber C, Koenen RR. Platelets and platelet-derived microparticles in vascular inflammatory disease. Inflamm Allergy Drug Targets. 2010;9:346–354. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
