Sensitivity of the Pig-a assay for detecting gene mutation in rats exposed acutely to strong clastogens
- PMID: 23677247
- PMCID: PMC3842102
- DOI: 10.1093/mutage/get022
Sensitivity of the Pig-a assay for detecting gene mutation in rats exposed acutely to strong clastogens
Abstract
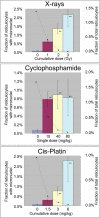
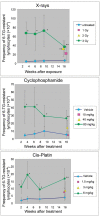
Clastogens are potential human carcinogens whose detection by genotoxicity assays is important for safety assessment. Although some endogenous genes are sensitive to the mutagenicity of clastogens, many genes that are used as reporters for in vivo mutation (e.g. transgenes) are not. In this study, we have compared responses in the erythrocyte Pig-a gene mutation assay with responses in a gene mutation assay that is relatively sensitive to clastogens, the lymphocyte Hprt assay, and in the reticulocyte micronucleus (MN) assay, which provides a direct measurement of clastogenicity. Male F344 rats were treated acutely with X-rays, cyclophosphamide (CP) and Cis-platin (Cis-Pt), and the frequency of micronucleated reticulocytes (MN RETs) in peripheral blood was measured 1 or 2 days later. The frequencies of CD59-deficient Pig-a mutant erythrocytes and 6-thioguanine-resistant Hprt mutant T-lymphocytes were measured at several times up to 16 weeks after the exposure. All three clastogens induced strong increases in the frequency of MN RETs, with X-rays and Cis-Pt producing near linear dose responses. The three agents also were positive in the two gene mutation assays although the assays detected them with different efficiencies. The Pig-a assay was more efficient in detecting the effect of Cis-Pt treatment, whereas the Hprt assay was more efficient for X-rays and CP. The results indicate that the erythrocyte Pig-a assay can detect the in vivo mutagenicity of clastogens although its sensitivity is variable in comparison with the lymphocyte Hprt assay.
Figures

References
-
- Bryce S. M., Bemis J. C., Dertinger S. D. (2008). In vivo mutation assay based on the endogenous Pig-a locus. Environ. Mol. Mutagen., 49, 256–264 - PubMed
-
- Miura D., Dobrovolsky V. N., Kasahara Y., Katsuura Y., Heflich R. H. (2008). Development of an in vivo gene mutation assay using the endogenous Pig-A gene: I. Flow cytometric detection of CD59-negative peripheral red blood cells and CD48-negative spleen T-cells from the rat. Environ. Mol. Mutagen., 49, 614–621 - PubMed
-
- Dobrovolsky V. N., Shaddock J. G., Mittelstaedt R. A., Manjanatha M. G., Miura D., Uchikawa M., Mattison D. R., Morris S. M. (2009). Evaluation of Macaca mulatta as a model for genotoxicity studies. Mutat. Res., 673, 21–28 - PubMed
-
- Dobrovolsky V. N., Elespuru R. K., Bigger C. A., Robison T. W., Heflich R. H. (2011). Monitoring humans for somatic mutation in the endogenous PIG-a gene using red blood cells. Environ. Mol. Mutagen., 52, 784–794 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

