Desmopressin melt improves response and compliance compared with tablet in treatment of primary monosymptomatic nocturnal enuresis
- PMID: 23677249
- PMCID: PMC3742424
- DOI: 10.1007/s00431-013-1992-9
Desmopressin melt improves response and compliance compared with tablet in treatment of primary monosymptomatic nocturnal enuresis
Abstract
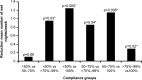
Primary nocturnal enuresis is a prevalent childhood condition that can persist into adulthood. Desmopressin is an antidiuretic available as orally disintegrating lyophilisate (melt) or solid tablet. Recent findings suggesting different food interactions and clinical characteristics, including compliance, between desmopressin melt and tablet motivated a post hoc analysis of a previously reported randomised, crossover study. The efficacy of desmopressin melt compared with tablet was evaluated using the International Children's Continence Society (ICCS) responder definitions. Compliance was further analysed using detailed criteria, and the association between efficacy and compliance was examined. In total, 221 patients aged 5-15 years, already receiving desmopressin tablets were randomised to the treatment sequence melt (120/240 μg)/tablet (0.2/0.4 mg) or tablet/melt in two consecutive 3-week periods. The probability of being a responder (partial or full) during either period was significantly more likely with desmopressin melt compared with tablet (odds ratio, 2.0; confidence intervals, 1.07-3.73; p = 0.03). There was no period effect on compliance in the tablet/melt sequence and no difference in the number of completely compliant patients in each formulation group; however, more patients were >75 % compliant in period 1 compared with period 2 in the melt/tablet sequence. Increased compliance was associated with greater reductions in the number of wet nights for both formulations.
Conclusions: Desmopressin melt, compared with tablet, improves the probability of being a responder. Switching from tablet to melt formulation increased patient compliance. Increased compliance was associated with increased efficacy. Switching to desmopressin melt may benefit patients with suboptimal responses to desmopressin tablet.
Trial registration: ClinicalTrials.gov NCT00209261.
Figures
Similar articles
-
Optimizing response to desmopressin in patients with monosymptomatic nocturnal enuresis.Pediatr Nephrol. 2017 Feb;32(2):217-226. doi: 10.1007/s00467-016-3376-7. Epub 2016 Apr 12. Pediatr Nephrol. 2017. PMID: 27071997 Free PMC article. Review.
-
A randomised comparison of oral desmopressin lyophilisate (MELT) and tablet formulations in children and adolescents with primary nocturnal enuresis.Int J Clin Pract. 2007 Sep;61(9):1454-60. doi: 10.1111/j.1742-1241.2007.01493.x. Epub 2007 Jul 26. Int J Clin Pract. 2007. PMID: 17655682 Clinical Trial.
-
Is there still a role for desmopressin in children with primary monosymptomatic nocturnal enuresis?: a focus on safety issues.Drug Saf. 2010 Apr 1;33(4):261-71. doi: 10.2165/11319110-000000000-00000. Drug Saf. 2010. PMID: 20297859
-
Pharmacokinetics of desmopressin administered as tablet and oral lyophilisate formulation in children with monosymptomatic nocturnal enuresis.Eur J Pediatr. 2014 Feb;173(2):223-8. doi: 10.1007/s00431-013-2108-2. Epub 2013 Aug 30. Eur J Pediatr. 2014. PMID: 23989967
-
Focus on desmopressin and enuresis: a review of literature.Minerva Urol Nefrol. 2016 Feb;68(1):14-9. Epub 2014 Jul 3. Minerva Urol Nefrol. 2016. PMID: 24990391 Review.
Cited by
-
Desmopressin as a hemostatic and blood sparing agent in bleeding disorders.Eur J Haematol. 2023 May;110(5):470-479. doi: 10.1111/ejh.13930. Epub 2023 Feb 12. Eur J Haematol. 2023. PMID: 36656570 Free PMC article. Review.
-
Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides.Int J Mol Sci. 2022 Mar 12;23(6):3068. doi: 10.3390/ijms23063068. Int J Mol Sci. 2022. PMID: 35328489 Free PMC article. Review.
-
The adverse effects of oral desmopressin lyophilisate (MELT): personal experience on enuretic children.Turk J Urol. 2018 Jan;44(1):51-55. doi: 10.5152/tud.2018.03285. Epub 2018 Jan 8. Turk J Urol. 2018. PMID: 29484228 Free PMC article.
-
An Integrated Paediatric Population PK/PD Analysis of dDAVP: How do PK Differences Translate to Clinical Outcomes?Clin Pharmacokinet. 2020 Jan;59(1):81-96. doi: 10.1007/s40262-019-00798-6. Clin Pharmacokinet. 2020. PMID: 31347012 Clinical Trial.
-
Optimizing response to desmopressin in patients with monosymptomatic nocturnal enuresis.Pediatr Nephrol. 2017 Feb;32(2):217-226. doi: 10.1007/s00467-016-3376-7. Epub 2016 Apr 12. Pediatr Nephrol. 2017. PMID: 27071997 Free PMC article. Review.
References
-
- De Guchtenaere A, Hoebeke P, Van Herzeele C, Dehoorne J, Raes A, Van Laecke E, De Bruyne P, Vande Walle J (2011) Pharmacokinetic characteristics of desmopressin oral lyophilisate (MELT) and tablet formulation in children. Neurourol Urodyn 30: Abstract 163
-
- Hjalmas K, Arnold T, Bower W, Caione P, Chiozza LM, von Gontard A, Han SW, Husman DA, Kawauchi A, Lackgren G, Lottmann H, Mark S, Rittig S, Robson L, Walle JV, Yeung CK. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004;171:2545–2561. doi: 10.1097/01.ju.0000111504.85822.b2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

