B lymphocyte "original sin" in the bone marrow enhances islet autoreactivity in type 1 diabetes-prone nonobese diabetic mice
- PMID: 23677466
- PMCID: PMC3679359
- DOI: 10.4049/jimmunol.1201359
B lymphocyte "original sin" in the bone marrow enhances islet autoreactivity in type 1 diabetes-prone nonobese diabetic mice
Abstract
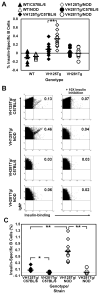
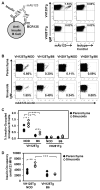
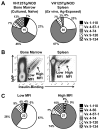
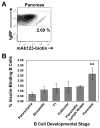
Effective central tolerance is required to control the large extent of autoreactivity normally present in the developing B cell repertoire. Insulin-reactive B cells are required for type 1 diabetes in the NOD mouse, because engineered mice lacking this population are protected from disease. The Cg-Tg(Igh-6/Igh-V125)2Jwt/JwtJ (VH125Tg) model is used to define this population, which is found with increased frequency in the periphery of NOD mice versus nonautoimmune C57BL/6 VH125Tg mice; however, the ontogeny of this disparity is unknown. To better understand the origins of these pernicious B cells, anti-insulin B cells were tracked during development in the polyclonal repertoire of VH125Tg mice. An increased proportion of insulin-binding B cells is apparent in NOD mice at the earliest point of Ag commitment in the bone marrow. Two predominant L chains were identified in B cells that bind heterologous insulin. Interestingly, Vκ4-57-1 polymorphisms that confer a CDR3 Pro-Pro motif enhance self-reactivity in VH125Tg/NOD mice. Despite binding circulating autoantigen in vivo, anti-insulin B cells transition from the parenchyma to the sinusoids in the bone marrow of NOD mice and enter the periphery unimpeded. Anti-insulin B cells expand at the site of autoimmune attack in the pancreas and correlate with increased numbers of IFN-γ-producing cells in the repertoire. These data identify the failure to cull autoreactive B cells in the bone marrow as the primary source of anti-insulin B cells in NOD mice and suggest that dysregulation of central tolerance permits their escape into the periphery to promote disease.
Figures



References
-
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. - PubMed
-
- Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 DK084246/DK/NIDDK NIH HHS/United States
- 5T32-AR059039/AR/NIAMS NIH HHS/United States
- P30 EY08126/EY/NEI NIH HHS/United States
- U01 HL065962/HL/NHLBI NIH HHS/United States
- 5T32-HL069765/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- R01-AI051448/AI/NIAID NIH HHS/United States
- R01 AI051448/AI/NIAID NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- T32 AR059039/AR/NIAMS NIH HHS/United States
- T32 HL069765/HL/NHLBI NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- K08 DK070924/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

