Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent
- PMID: 23677473
- PMCID: PMC3756559
- DOI: 10.4049/jimmunol.1202583
Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent
Abstract
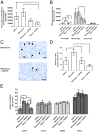
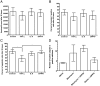
In Dictyostelium discoideum, AprA is a secreted protein that inhibits proliferation and causes chemorepulsion of Dictyostelium cells, yet AprA has little sequence similarity to any human proteins. We found that a predicted structure of AprA has similarity to human dipeptidyl peptidase IV (DPPIV). DPPIV is a serine protease present in extracellular fluids that cleaves peptides with a proline or alanine in the second position. In Insall chambers, DPPIV gradients below, similar to, and above the human serum DPPIV concentration cause movement of human neutrophils away from the higher concentration of DPPIV. A 1% DPPIV concentration difference between the front and back of the cell is sufficient to cause chemorepulsion. Neutrophil speed and viability are unaffected by DPPIV. DPPIV inhibitors block DPPIV-mediated chemorepulsion. In a murine model of acute respiratory distress syndrome, aspirated bleomycin induces a significant increase in the number of neutrophils in the lungs after 3 d. Oropharyngeal aspiration of DPPIV inhibits the bleomycin-induced accumulation of mouse neutrophils. These results indicate that DPPIV functions as a chemorepellent of human and mouse neutrophils, and they suggest new mechanisms to inhibit neutrophil accumulation in acute respiratory distress syndrome.
Figures




Similar articles
-
Protease activated-receptor 2 is necessary for neutrophil chemorepulsion induced by trypsin, tryptase, or dipeptidyl peptidase IV.J Leukoc Biol. 2018 Jan;103(1):119-128. doi: 10.1002/JLB.3A0717-308R. Epub 2017 Dec 11. J Leukoc Biol. 2018. PMID: 29345066 Free PMC article.
-
Functional similarities between the dictyostelium protein AprA and the human protein dipeptidyl-peptidase IV.Protein Sci. 2017 Mar;26(3):578-585. doi: 10.1002/pro.3107. Epub 2017 Feb 15. Protein Sci. 2017. PMID: 28028841 Free PMC article.
-
Using Dictyostelium to Develop Therapeutics for Acute Respiratory Distress Syndrome.Front Cell Dev Biol. 2021 Jul 19;9:710005. doi: 10.3389/fcell.2021.710005. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34350188 Free PMC article. Review.
-
Role of the neutrophil chemorepellent soluble dipeptidyl peptidase IV in decreasing inflammation in a murine model of arthritis.Arthritis Rheumatol. 2015 Oct;67(10):2634-8. doi: 10.1002/art.39250. Arthritis Rheumatol. 2015. PMID: 26138693 Free PMC article.
-
Potential role of dipeptidyl peptidase IV in the pathophysiology of heart failure.Int J Mol Sci. 2015 Feb 16;16(2):4226-49. doi: 10.3390/ijms16024226. Int J Mol Sci. 2015. PMID: 25690036 Free PMC article. Review.
Cited by
-
Identification of novel proteins in the Dictyostelium discoideum chemorepulsion pathway using REMI.MicroPubl Biol. 2022 May 5;2022:10.17912/micropub.biology.000557. doi: 10.17912/micropub.biology.000557. eCollection 2022. MicroPubl Biol. 2022. PMID: 35622529 Free PMC article.
-
A phosphatidylinositol phosphate kinase inhibits Ras activation and regulates chemorepulsion in Dictyostelium discoideum.J Cell Sci. 2023 Jul 15;136(14):jcs260541. doi: 10.1242/jcs.260541. Epub 2023 Jul 27. J Cell Sci. 2023. PMID: 37259831 Free PMC article.
-
An endogenous chemorepellent directs cell movement by inhibiting pseudopods at one side of cells.Mol Biol Cell. 2019 Jan 15;30(2):242-255. doi: 10.1091/mbc.E18-09-0562. Epub 2018 Nov 21. Mol Biol Cell. 2019. PMID: 30462573 Free PMC article.
-
The sialidase NEU3 promotes pulmonary fibrosis in mice.Respir Res. 2022 Aug 23;23(1):215. doi: 10.1186/s12931-022-02146-y. Respir Res. 2022. PMID: 35999554 Free PMC article.
-
The Use of Diffusion Calculations and Monte Carlo Simulations to Understand the Behavior of Cells in Dictyostelium Communities.Comput Struct Biotechnol J. 2019 Jun 8;17:684-688. doi: 10.1016/j.csbj.2019.06.002. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 31303972 Free PMC article. Review.
References
-
- Baker MD, Wolanin PM, Stock JB. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. - PubMed
-
- Leonard EJ, Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]) Am J Respir Cell Mol Biol. 1990;2:479–486. - PubMed
-
- Crossley LJ. Neutrophil activation by fMLP regulates FOXO (forkhead) transcription factors by multiple pathways, one of which includes the binding of FOXO to the survival factor Mcl-1. J Leukoc Biol. 2003;74:583–592. - PubMed
-
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

