Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity
- PMID: 23677477
- PMCID: PMC4084782
- DOI: 10.4049/jimmunol.1300022
Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity
Abstract
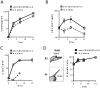
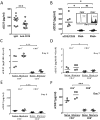
The interaction between CD27 and its ligand, CD70, has been implicated in regulating cellular immune responses to cancer. In this article, we report on the role of soluble CD27 (sCD27) in T cell activation and its elevation in the serum of cancer patients after immunotherapy. In vitro, sCD27 is preferentially derived from activated CD4(+) T cells. Adding sCD27 to stimulated PBMCs increases T cell activation and proliferation, and is associated with the immunologic synapse-related proteins myosin IIA, high mobility group box 1, and the TCR Vβ-chain. The pool of serum sCD27 is shown to be greater in healthy donors than in cancer patients. However, metastatic cancer patients treated with immunotherapy showed a significant increase in the serum sCD27-pool posttherapy (p < 0.0005); there was also an increased trend toward an association between enhanced sCD27-pool posttherapy and overall survival (p = 0.022). The identification of sCD27 as an immune modulator associated with enhanced human T cell activation in vitro and in vivo provides a rationale for developing new immunotherapeutic strategies aimed at enhancing sCD27 for treating cancer and potentially other diseases.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Expression of soluble CD27 in extranodal natural killer/T-cell lymphoma, nasal type: potential as a biomarker for diagnosis and CD27/CD70-targeted therapy.Cancer Immunol Immunother. 2023 Jul;72(7):2087-2098. doi: 10.1007/s00262-023-03394-7. Epub 2023 Feb 22. Cancer Immunol Immunother. 2023. PMID: 36811663 Free PMC article.
-
CD27 expression on lymphocyte and sCD27 levels in children with asthma.Allergol Immunopathol (Madr). 2010 Nov-Dec;38(6):327-32. doi: 10.1016/j.aller.2010.02.008. Epub 2010 Aug 30. Allergol Immunopathol (Madr). 2010. PMID: 20800938
-
Soluble CD27 as a predictive biomarker for intra-tumoral CD70/CD27 interaction in nasopharyngeal carcinoma.Cancer Sci. 2024 Apr;115(4):1073-1084. doi: 10.1111/cas.16079. Epub 2024 Jan 27. Cancer Sci. 2024. PMID: 38279834 Free PMC article.
-
Control of lymphocyte function through CD27-CD70 interactions.Semin Immunol. 1998 Dec;10(6):491-9. doi: 10.1006/smim.1998.0154. Semin Immunol. 1998. PMID: 9826582 Review.
-
CD27/CD70 interactions regulate T dependent B cell differentiation.Immunol Res. 2000;21(1):23-30. doi: 10.1385/IR:21:1:23. Immunol Res. 2000. PMID: 10803880 Review.
Cited by
-
Role of ADAM10 and ADAM17 in Regulating CD137 Function.Int J Mol Sci. 2021 Mar 8;22(5):2730. doi: 10.3390/ijms22052730. Int J Mol Sci. 2021. PMID: 33800462 Free PMC article.
-
A phase I study of recombinant (r) vaccinia-CEA(6D)-TRICOM and rFowlpox-CEA(6D)-TRICOM vaccines with GM-CSF and IFN-α-2b in patients with CEA-expressing carcinomas.Cancer Immunol Immunother. 2016 Nov;65(11):1353-1364. doi: 10.1007/s00262-016-1893-7. Epub 2016 Aug 31. Cancer Immunol Immunother. 2016. PMID: 27581603 Free PMC article. Clinical Trial.
-
Association of baseline soluble immune checkpoints with the risk of relapse in PR3-ANCA vasculitis following induction of remission.Ann Rheum Dis. 2023 Feb;82(2):253-261. doi: 10.1136/ard-2022-222479. Epub 2022 Aug 16. Ann Rheum Dis. 2023. PMID: 35973802 Free PMC article. Clinical Trial.
-
Immune correlates of clinical parameters in patients with HPV-associated malignancies treated with bintrafusp alfa.J Immunother Cancer. 2022 Apr;10(4):e004601. doi: 10.1136/jitc-2022-004601. J Immunother Cancer. 2022. PMID: 35418484 Free PMC article.
-
CD27+ microparticle interactions and immunoregulation of CD4+ T lymphocytes.Front Immunol. 2023 Mar 9;14:1043255. doi: 10.3389/fimmu.2023.1043255. eCollection 2023. Front Immunol. 2023. PMID: 36969173 Free PMC article.
References
-
- Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. - PubMed
-
- Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–499. - PubMed
-
- Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA. CD70 represents the human ligand for CD27. Int Immunol. 1994;6:477–480. - PubMed
-
- Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J Immunol. 1997;159:4959–4965. - PubMed
-
- Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

